Unchanged protein binding of penbutolol in renal insufficiency: a possible role of carbamylation.
C Aguirre, R Calvo, J M Rodriguez-Sasiain
文献索引:Int. J. Clin. Pharmacol. Ther. Toxicol. 31(1) , 31-4, (1993)
全文:HTML全文
摘要
The effect of in vitro carbamylation of serum protein with potassium cyanate on protein binding of penbutolol, a basic agent exclusively bound to alpha 1 acid glycoprotein (AAG), was investigated. Carbamylation of serum resulted in a weak increase on free fraction of penbutolol (4.45 +/- 0.54% before carbamylation vs 5.66 +/- 0.40% after; p < 0.025). Parallelly, potassium cyanate added to pure AAG and incubated for 90 min induced carbamylation of this protein (38 mumoles of 14C cyanate incorporated per gram of protein). A study in serum from patients with chronic renal disease (pre and postdialysis) showed no changes in protein binding of penbutolol, although AAG levels were significantly higher. However, Scatchard [1949] plot for penbutolol binding to serum from renal patients (both pre and postdialysis) showed a decrease in affinity constant (nKa = 11.13 x 10(5) M-1 in healthy volunteers, vs 5.56 x 10(5) M-1 in patients before dialysis and 4.57 x 10(5) M-1 after dialysis). We concluded that carbamylation of serum AAG in uremic patients could explain, in part, the absence of changes in protein binding of any basic drugs in this pathological condition. It appears that a decreased affinity constant could balance the effect of increased AAG levels.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
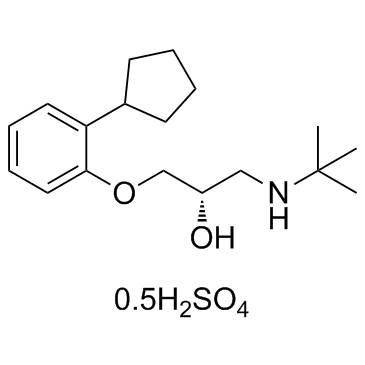 |
甲醇中喷布特罗溶液标准物质
CAS:38363-32-5 |
C18H29NO2.1/2H2O4S |
|
5-HT receptor subtypes involved in the spinal antinociceptiv...
2001-11-30 [Eur. J. Pharmacol. 432(1) , 1-7, (2001)] |
|
Autoreceptors remain functional after prolonged treatment wi...
1999-07-24 [Brain Res. 835(2) , 224-8, (1999)] |
|
Affinity of (+/-)-pindolol, (-)-penbutolol, and (-)-tertatol...
2000-08-01 [J. Neurochem. 75(2) , 755-62, (2000)] |
|
The antiaggressive potency of (-)-penbutolol involves both 5...
1996-02-15 [Eur. J. Pharmacol. 297(1-2) , 1-8, (1996)] |
|
High-performance liquid chromatography with chemiluminescenc...
2001-06-15 [J. Chromatogr. B. Biomed. Sci. Appl. 757(2) , 229-35, (2001)] |