In vitro inhibitory effect of tetrahydrocurcuminoids on Fusarium proliferatum growth and fumonisin B₁ biosynthesis.
Véronique Coma, Elise Portes, Christian Gardrat, Florence Richard-Forget, Alain Castellan
文献索引:Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 28(2) , 218-25, (2011)
全文:HTML全文
摘要
Many plant pathogens produce toxic metabolites when growing on food and feed. Some antioxidative components seem to prevent fungal growth and mycotoxin formation. Recently, we synthesized a new class of powerful antioxidative compounds, i.e. tetrahydrocurcuminoids, and its structure/antioxidant activity relationships have been established. The South West of France produces large amounts of corn, which can be infected by Fusarium species, particularly F. proliferatum. In this context, the efficiency of tetrahydrocurcuminoids, which can be obtained from natural curcuminoids, was investigated to control in vitro the growth of F. proliferatum and the production of its associated mycotoxin, fumonisin B₁. The relation between structure and antifungal activity was studied. Tetrahydrocurcumin (THC1), with two guaiacyl phenolic subunits, showed the highest inhibitory activity (measured as radial growth on agar medium) against the F. proliferatum development (67% inhibition at a concentration of 13.6 µmol ml⁻¹). The efficiencies of THC2 (36% at a concentration of 11.5 µmol ml⁻¹), which contains syringyl phenolic units, and THC3 (30% at a concentration of 13.6 µmol ml⁻¹), which does not have any substituent on the aromatic rings, were relatively close. These results indicate that the simultaneous presence of guaiacyl phenols and the enolic function of the β-diketone moiety play an important role in the inhibition mechanisms. The importance of this combination was confirmed using n-propylguaiacol and acetylacetone as molecular models. Under the same conditions, ferulic acid and eugenol, other natural phenolic antioxidants, were less efficient in inhibiting fungal growth. THC1 also reduced fumonisin B₁ production in liquid medium by approximately 35, 50 and 75% at concentrations of 0.8, 1.3, and 1.9 µmol ml⁻¹, respectively. These very low inhibitory concentrations show that tetrahydrocurcuminoids could be one of the most promising biobased molecules for the control of mycotoxinogen fungal strains.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
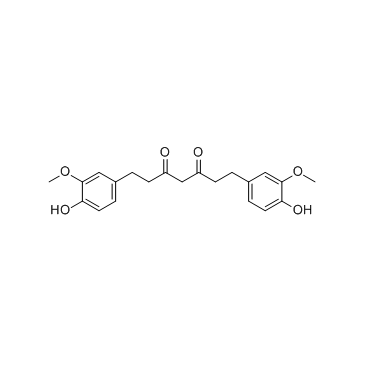 |
四氢姜黄素
CAS:36062-04-1 |
C21H24O6 |
|
Structure-activity relationship analysis of curcumin analogu...
2013-11-01 [FEBS J. 280(22) , 5829-40, (2013)] |
|
Differential cellular uptake and metabolism of curcuminoids ...
2014-07-14 [Br. J. Nutr. 112(1) , 8-14, (2014)] |
|
Synthesis of quinoline derivatives of tetrahydrocurcumin and...
2013-01-15 [Food Chem. 136(2) , 650-8, (2013)] |
|
Antioxidant combinations protect oral fibroblasts against me...
2013-03-01 [Arch. Oral Biol. 58(3) , 299-310, (2013)] |
|
Inhibition of Ca(2+) release-activated Ca(2+) channel (CRAC)...
2012-11-09 [Biochem. Biophys. Res. Commun. 428(1) , 56-61, (2012)] |