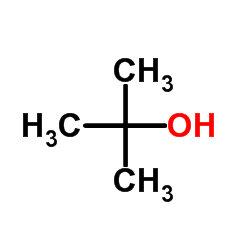
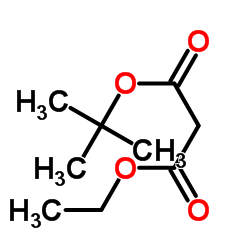
|
~41% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~44% |
|
~97% |
|
~99% |
|
~10% |
|
~53% |
|
~67% |
|
~48% |
|
~37% |
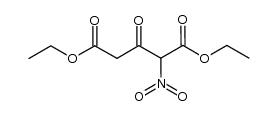
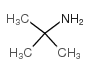
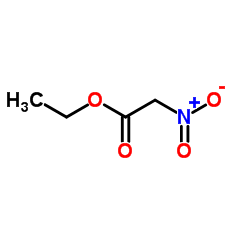
![Propanoic acid,3-[(1,1-dimethylethyl)amino]-3-oxo-, ethyl ester结构式](https://image.chemsrc.com/caspic/216/17797-87-4.png)



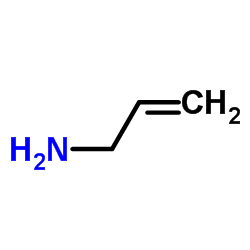
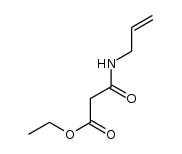
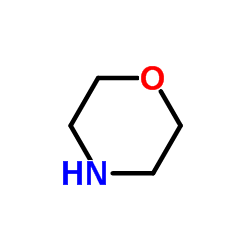

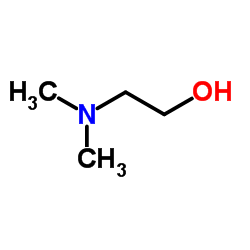
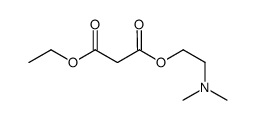
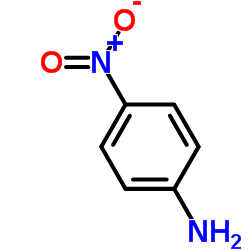
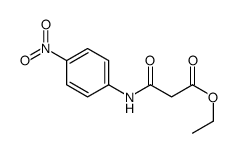
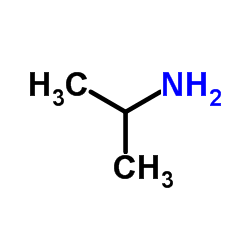
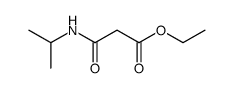
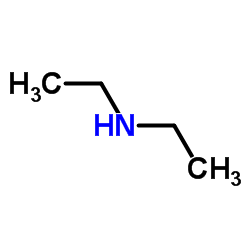
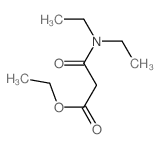

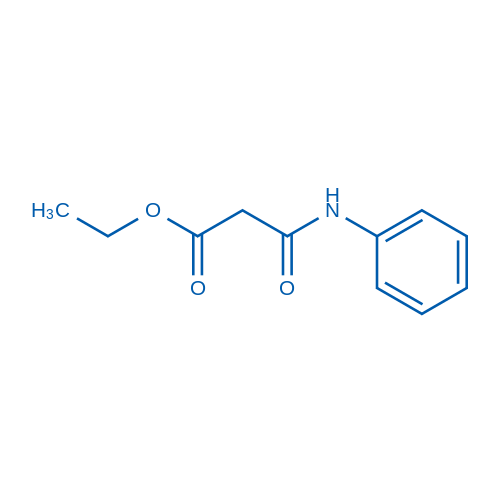

![3-methylsulfanyl-1,6-diphenyl-1,4-dihydro-[1,2,4,5]tetrazine结构式](https://image.chemsrc.com/caspic/105/50780-97-7.png)