| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
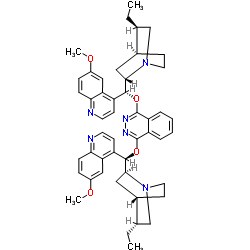 |
氢化奎宁 1,4-(2,3-二氮杂萘)二醚
CAS:140924-50-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氢化奎宁 1,4-(2,3-二氮杂萘)二醚
CAS:140924-50-1 |