Production of biologically active recombinant avidin in baculovirus-infected insect cells.
K J Airenne, C Oker-Blom, V S Marjomäki, E A Bayer, M Wilchek, M S Kulomaa
文献索引:Protein Expr. Purif. 9(1) , 100-8, (1997)
全文:HTML全文
摘要
An efficient lepidopteran insect cell system was established for the expression of a recombinant form of chicken egg-white avidin. The gene product was obtained in both secreted and intracellular forms, and biologically active recombinant avidin was isolated using affinity chromatography on an iminobiotin-agarose column. Similar to the known quaternary structure of the native egg-white protein, the purified recombinant protein was glycosylated and assembled mainly into tetramers. Like native avidin, the recombinant tetramer also exhibited a high level of thermostability, and was further stabilized upon binding biotin. The biotin-binding and structural properties of the recombinant avidin are thus similar to those of the natural egg-white protein, and the insect system is appropriate both for future site-directed mutagenesis studies and for the production of avidin fusion proteins.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
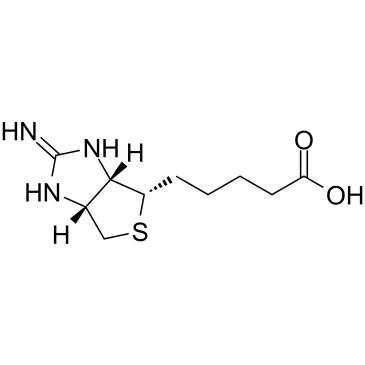 |
2-亚氨基生物素
CAS:13395-35-2 |
C10H17N3O2S |
|
Influence of different classes of NO synthase inhibitors in ...
1997-10-01 [Naunyn Schmiedebergs Arch. Pharmacol. 356(4) , 488-94, (1997)] |
|
Intermolecular forces and energies between ligands and recep...
1994-10-14 [Science 266(5183) , 257-9, (1994)] |
|
Effects of selective nitric oxide synthase inhibition on IGF...
2002-01-01 [Dev. Neurosci. 24(5) , 396-404, (2002)] |
|
Pharmacological interventions in the newborn piglet in the f...
2002-11-01 [Exp. Brain Res. 147(2) , 200-8, (2002)] |
|
Effects of hydrogen peroxide on the electrochemical decompos...
2007-11-01 [J. Colloid. Interface Sci. 315(1) , 396-9, (2007)] |