Biotransformation of isoimperatorin and imperatorin by Glomerella cingulata and beta-secretase inhibitory activity.
Shinsuke Marumoto, Mitsuo Miyazawa
文献索引:Bioorg. Med. Chem. 18(1) , 455-9, (2010)
全文:HTML全文
摘要
Biotransformation studies conducted on the furanocoumarins isoimperatorin (1) and imperatorin (3) have revealed that 1 was metabolized by Glomerella cingulata to give the corresponding reduced acid, 6,7-furano-5-prenyloxy hydrocoumaric acid (2), and 3 was transformed by G. cingulata to give the dealkylated metabolite, xanthotoxol (4) in high yields (83% and 81%), respectively. The structures of the new compound 2 have been established on the basis of spectral data. The metabolites 2 and 4 were tested for the beta-secretase (BACE1) inhibitory activity in vitro, and metabolite 2 slightly inhibited the beta-secretase activity with an IC(50) value of 185.6+/-6.8 microM. The metabolite 4 was less potent activity than compounds 1-3. In addition, methyl ester (2Me), methyl ether (2a) and methyl ester and ether (2aMe) of 2 were synthesized, and investigated for the ability to inhibit beta-secretase. Compound 2aMe exhibited the best beta-secretase inhibitory activity at the IC(50) value 16.2+/-1.2 microM and found to be the 2aMe showed competitive mode of inhibition against beta-secretase with K(i) value 11.3+/-2.8 microM.Copyright (c) 2009 Elsevier Ltd. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
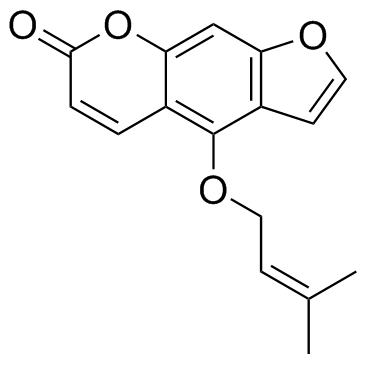 |
异欧前胡素
CAS:482-45-1 |
C16H14O4 |
|
In vitro activity of isoimperatorin, alone and in combinatio...
2014-04-01 [Lett. Appl. Microbiol. 58(4) , 344-9, (2014)] |
|
Study on pharmacokinetics and tissue distribution of pteryxi...
2012-07-01 [Biomed. Chromatogr. 26(7) , 802-7, (2012)] |
|
Ethyl acetate extract from Angelica Dahuricae Radix inhibits...
2007-04-01 [Pharmacol. Res. 55(4) , 263-70, (2007)] |
|
The effects of isoimperatorin isolated from Angelicae dahuri...
2008-02-01 [Arch. Pharm. Res. 31(2) , 210-5, (2008)] |
|
Development and validation of a gas chromatography-mass spec...
2007-06-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 852(1-2) , 473-8, (2007)] |