Solution structure of succinylacetone, an unsymmetrical beta-diketone, as studied by 13C NMR and GIAO-DFT calculations.
Dominika Bal, Anna Kraska-Dziadecka, Adam Gryff-Keller
文献索引:J. Org. Chem. 74(22) , 8604-9, (2009)
全文:HTML全文
摘要
The enolization degrees of succinylacetone, an important heme biosynthesis inhibitor, have been determined in CDCl(3) and water solutions using (1)H NMR. The solution structures of SA have been investigated using a combined NMR/theoretical [GIAO DFT PBE1PBE/6-311++G(2d, p) PCM] approach. The populations of both enolic forms undergoing enol-enol equilibriums for SA and a series of unsymmetrical beta-diketones have been established by a quantitative comparison of the experimental (13)C NMR chemical shifts and calculated shielding constants. Moreover, using the same method and considering various trial structures differing in conformation and/or hydration of neutral SA molecule as well as its monoanion and dianion the structures of the most abundant species being present in the investigated water solutions have been deduced.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
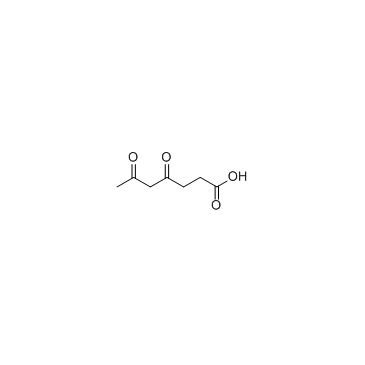 |
4,6-二氧代庚酸
CAS:51568-18-4 |
C7H10O4 |
|
The heme exporter Flvcr1 regulates expansion and differentia...
2015-06-01 [Haematologica 100 , 720-9, (2015)] |
|
Heme Oxygenase-1 Regulation of Matrix Metalloproteinase-1 Ex...
2015-09-15 [J. Immunol. 195 , 2763-73, (2015)] |
|
Single dose NTBC-treatment of hereditary tyrosinemia type I.
2012-09-01 [J. Inherit. Metab. Dis. 35(5) , 831-6, (2012)] |
|
LC-MS/MS method for simultaneous determination on a dried bl...
2012-01-17 [Anal. Chem. 84(2) , 1184-8, (2012)] |
|
Clinical, biochemical, and genetic analysis of a Korean neon...
2009-01-01 [Clin. Chem. Lab Med. 47(8) , 930-3, (2009)] |