Protein kinase C modulation of fibronectin matrix assembly.
C E Somers, D F Mosher
文献索引:J. Biol. Chem. 268(30) , 22277-80, (1993)
全文:HTML全文
摘要
Fibroblasts have cell surface sites that mediate the assembly of fibronectin (Fn) into the extracellular matrix. Treatment of fibroblasts with kinase inhibitors (ML-7, H7, HA1004, calphostin C, and staurosporine) resulted in the rapid decrease in the binding of 125I-labeled plasma Fn and iodinated amino-terminal fragments of Fn. The dose responses of the four inhibitors suggest that the target kinase is protein kinase C (PKC) rather than the cyclic AMP- or cyclic GMP-dependent kinases. Three different fibroblastic cells were similarly affected. The inhibition was rapid and reversible and could not be overcome by increasing concentrations of Fn. Treatment of fibroblasts with phorbol esters and other agents that activate PKC resulted in increased amounts of 125I-labeled Fn binding to the cell surface. These results imply that Fn matrix assembly is modulated by PKC-mediated phosphorylation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
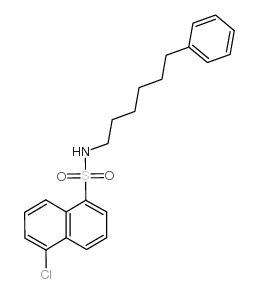 |
5-氯-N-(6-苯基己基)-1-萘磺酰胺
CAS:102649-78-5 |
C22H24ClNO2S |
|
Stimulation of choline transport in cultured cells induced b...
1988-01-01 [Oncology 45(4) , 326-30, (1988)] |
|
N-(6-phenylhexyl)-5-chloro-1-naphthalenesulfonamide is one o...
1986-11-28 [Biochim. Biophys. Acta 889 , 236, (1986)] |
|
N-(6-phenylhexyl)-5-chloro-1-naphthalenesulfonamide, a novel...
1986-07-29 [Biochemistry 25 , 4179, (1986)] |
|
Evaluation of Salmonella live vaccines with chromosomal expr...
2009-06-08 [Vaccine 27 , 3780-7, (2009)] |
|
Manipulation of phototransductive membrane turnover by crab ...
[J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 170(2) , 189-99, (1992)] |