Polymorphic behavior in protein-surfactant mixtures: the water-bovine serum albumin-sodium taurodeoxycholate system.
Barbara Orioni, Mauro Roversi, Camillo La Mesa, Fioretta Asaro, Giorgio Pellizer, Gerardino D'Errico
文献索引:J. Phys. Chem. B 110 , 12129, (2006)
全文:HTML全文
摘要
Mixtures containing water, bovine serum albumin (BSA), and sodium taurodeoxycholate (NaTDC), a component of the bile in mammals, have been investigated in a wide range of composition and pH. Depending on the concentration of both solutes and the pH, solutions, precipitates, and gels are formed. Under spontaneous pH conditions, the transport properties in dilute solutions indicate the occurrence of significant interactions between BSA and the surfactant. Conversely, acidic media favor the formation of nonsoluble protein-surfactant complexes, with subsequent precipitation. The nucleation kinetics of the protein-surfactant complexes in solid form and the related precipitation processes can be slow or fast, depending on the overall solute content and the mole ratio. At high concentrations, a gel, extending on both sides of the charge neutralization line, and two-phase regions are observed. Gels shrink in open air and swell in the presence of excess water. Depending on concentration and temperature, the gels transform from an essentially liquidlike behavior to that peculiar to true gels (when G' > or = G''). The thermal gelation threshold, the temperature above which G' > or = G'', depends on BSA and NaTDC content and is concomitant to moderate heat effects, inferred by differential scanning calorimetry (DSC). The above data also indicate that the protein thermal denaturation in the gel is shifted to higher temperatures compared to water. Such a stabilizing effect is presumably related to the occurrence of both electrostatic and hydrophobic interactions with NaTDC. Water self-diffusion in the gels is slightly slower than that in the bulk and poorly sensitive to composition: it is about 65% the value of neat H2O in a wide concentration range, irrespective of the BSA, or NaTDC, concentration. A peculiar behavior is also observed in 23Na longitudinal and transverse relaxation rates. The T1 and T2 values, measured at 105.75 MHz on BSA-NaTDC gels, indicate that the motions determining the NMR relaxation of the sodium ions in the hydration layer of the protein-surfactant aggregates are not slow, having frequencies comparable with the Larmor one. The above properties, especially the rheological and the spectroscopic ones, are important for understanding the behavior of gels based on protein-surfactant mixtures.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
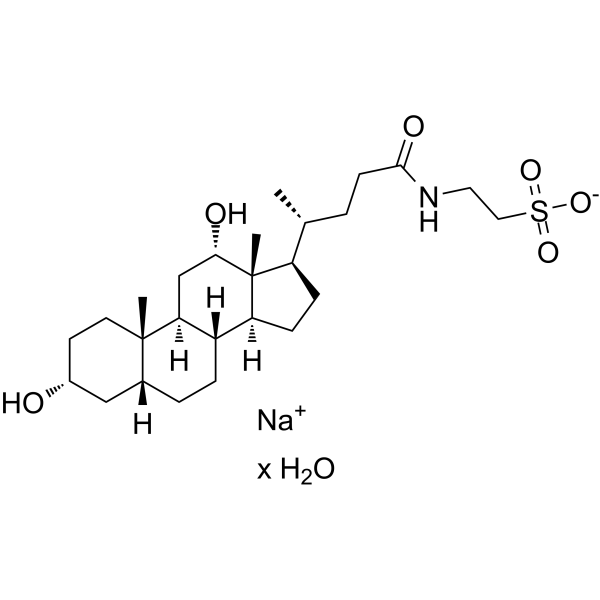 |
牛磺酸脱氧胆酸钠 水合物
CAS:207737-97-1 |
C26H44NNaO6S |
|
Shiga toxin glycosphingolipid receptors of Vero-B4 kidney ep...
2015-12-01 [J. Lipid Res. 56 , 2322-36, (2015)] |
|
Bile acids repress hypoxia-inducible factor 1 signaling and ...
2014-09-01 [Infect. Immun. 82(9) , 3531-41, (2014)] |
|
Regulation of host weight gain and lipid metabolism by bacte...
2014-05-20 [Proc. Natl. Acad. Sci. U. S. A. 111(20) , 7421-6, (2014)] |
|
Effect of submicellar concentrations of conjugated and uncon...
2011-11-15 [Langmuir 27 , 13461, (2011)] |
|
Short-term feedback regulation of bile salt uptake by bile s...
2012-12-01 [Hepatology 56 , 2387, (2012)] |