| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
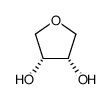 |
1,4-酐-赤藓糖醇
CAS:4358-64-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
1,4-酐-赤藓糖醇
CAS:4358-64-9 |