5-methoxy-2-methyl-3-indole acetic acid, a metabolite and alkali hydrolysis product of indomethacin, inhibits platelet aggregation, in vivo.
W I Rosenblum, F El-Sabban, G H Nelson
文献索引:Prostaglandins 21(4) , 667-73, (1981)
全文:HTML全文
摘要
Previous studies suggested that one of the hydrolysis products of indomethacin, either 4-chlorobenzoic acid or 5-methoxy-2-methyl-3-indole acetic acid, can inhibit platelet aggregation in vivo. If correct, this hypothesis explains the apparent action of indomethacin dissolved at high pH where hydrolysis rapidly occurs. Moreover, if the indole is the active product, the hypothesis in addition suggests a possible role for the indole following the use of indomethacin dissolved at lower pH, since the indole is also an important natural metabolite of indomethacin in man. We induced platelet aggregation in cerebral and mesenteric microvessels and inhibited this aggregation with the indole (25 mg/kg i.p. one hour before test). The 4-chlorobenzoic acid was inactive. The indole had a modest but statistically significant inhibitory effect in vitro, when added to platelet rich plasma stimulated to aggregate by arachidonic acid (0.5 mM). Thus the action of indomethacin dissolved at high pH is explained, and a pharmacologic action of a metabolite of indomethacin becomes a possibility.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
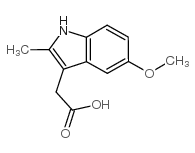 |
5-甲氧基-2-甲基-3-吲哚乙酸
CAS:2882-15-7 |
C12H13NO3 |
|
HPLC analysis of indomethacin and its impurities in capsule ...
1982-07-01 [J. Pharm. Sci. 71(7) , 828-30, (1982)] |
|
Accurate assignment of ethanol origin in postmortem urine: l...
2004-06-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 805(2) , 223-34, (2004)] |
|
Simultaneous densitometric determination of indomethacin and...
2001-01-01 [J. AOAC Int. 84(6) , 1703-7, (2001)] |
|
Axonal injury-dependent induction of the peripheral benzodia...
2004-08-01 [Eur. J. Neurosci. 20(3) , 671-83, (2004)] |
|
Expression of mitochondrial benzodiazepine receptor and its ...
1994-09-01 [Cell Growth Differ. 5(9) , 1005-14, (1994)] |