| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
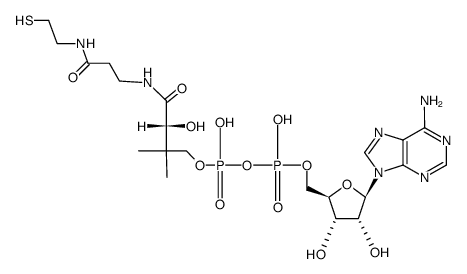 |
3'-去磷酸辅酶 A
CAS:3633-59-8 |
|
 |
柠檬酸裂解酶 来源于肺炎克雷伯氏杆菌
CAS:9012-83-3 |