Purification of an acid proteinase from Aspergillus saitoi and determination of peptide bond specificity.
N Tanaka, M Takeuchi, E Ichishima
文献索引:Biochim. Biophys. Acta 485(2) , 406-16, (1977)
全文:HTML全文
摘要
The specificity and mode of action of an acid proteinase (EC 3.4.23.6) from Aspergillus saitoi were investigated with oxidized B-chain of insulin, angiotensin II and bradykinin. Further purification of acid proteinase was performed with N,O-dibenzyloxycarbonyl-tyrosine hexamethylene-diamino-Sepharose 4B affinity chromatography and isoelectric focusing. The purified enzyme was free of any other proteolytic activity demonstrated in Asp. saitoi. Acid proteinase from Asp. saitoi hydrolyzed primarily two peptide bonds in the oxidized B-chain of insulin, the Leu(15)-Tyr(16) bond and the Phe(24)-Phe(25) bond. Additional cleavages of the bonds His(10)-Leu(11), Ala(14)-Leu(15) and Tyr(16)-Leu(17) were also noted. Primary splitting sites at Leu(15)-Tyr(16) and Phe(24-)-Phe(25) with acid proteinase from Asp. saitoi were identical with those reported in the work of cathepsin D (EC 3.4.23.5) from human erythrocyte. Hydrolysis of angiotensin II was observed at the Tyr(4)-Ile(5) bond. In conclusion, peptide bonds which have a hydrophobic amino acid such as phenylalanine, tyrosine, leucine and isoleucine in the P'1 position (as defined by Berger and Schechter, [29]) are preferentially cleaved by the trypsinogenactivating acid proteinase from Asp. saitoi.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
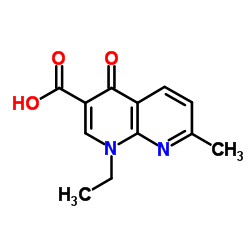 |
酸性蛋白酶
CAS:9025-49-4 |
C12H12N2O3 |
|
[Modification of two tyrosine residues in aspergillopepsin A...
1981-02-01 [Biokhimiia 46(2) , 369-75, (1981)] |
|
Sequences from the aspergillopepsin PEP gene of Aspergillus ...
1999-08-15 [FEMS Immunol. Med. Microbiol. 25(3) , 255-64, (1999)] |
|
The site of diazoacetyl inhibitor attachment to acid protein...
1972-11-15 [Biochem. Biophys. Res. Commun. 49(4) , 1075-81, (1972)] |
|
Isolation and characterization of mutants of Aspergillus nig...
1992-08-01 [Mol. Gen. Genet. 234(2) , 332-6, (1992)] |
|
Molecular cloning of a cDNA for proctase B from Aspergillus ...
1995-05-01 [Biosci. Biotechnol. Biochem. 59(5) , 954-5, (1995)] |