The rational use of hydrophobic effect-based recognition in molecularly imprinted polymers.
S A Piletsky, H S Andersson, I A Nicholls
文献索引:J. Mol. Recognit. 11(1-6) , 94-7, (1998)
全文:HTML全文
摘要
A novel molecularly imprinted polymer (MIP) system selective for D-phenylalanine is described where polymerization is performed in aqueous solution. The unique polymer system comprises a hydrophobic moiety-selective functional monomer, polymerizable beta-cyclodextrin, an electrostatic interacting functional monomer, 2-acryloylamido-2-methylpropane sulfonic acid (AMPSA), and the crosslinking agent N,N'-diacryloylpiperazine. Chromatographic evaluation of polymer-ligand recognition characteristics demonstrated ligand selectivity by the MIP and that optimal recognition was achieved through a balance of hydrophobic and electrostatic ligand-polymer interactions, indicating that recognition in these systems is regulated by enthalpy-entropy compensation. The imprinting effect was shown to be sufficient to reverse the inherent selectivity of cyclodextrin for L-phenylalanine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
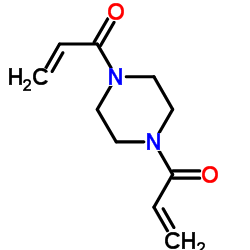 |
1,4-二丙烯酰基哌嗪
CAS:6342-17-2 |
C10H14N2O2 |
|
Peroxiredoxin I protein, a potential biomarker of hydronephr...
2014-06-01 [J. Pediatr. Urol. 10(3) , 474-81, (2014)] |
|
Urine proteome analysis in Dent's disease shows high selecti...
2016-01-01 [J. Proteomics 130 , 26-32, (2015)] |
|
ECM stiffness primes the TGFβ pathway to promote chondrocyte...
2012-09-01 [Mol. Biol. Cell 23 , 3731-42, (2012)] |
|
Application of MeCAT-Click labeling for protein abundance ch...
2016-03-16 [J. Proteomics 136 , 68-76, (2016)] |
|
Methods for increasing the resolution of two-dimensional pro...
1988-09-01 [Anal. Biochem. 173 , 424, (1988)] |