Prospective trial of customized adherence enhancement plus long-acting injectable antipsychotic medication in homeless or recently homeless individuals with schizophrenia or schizoaffective disorder.
Martha Sajatovic, Jennifer Levin, Luis F Ramirez, David Y Hahn, Curtis Tatsuoka, Christopher S Bialko, Kristin A Cassidy, Edna Fuentes-Casiano, Tiffany D Williams
文献索引:J. Clin. Psychiatry 74(12) , 1249-55, (2013)
全文:HTML全文
摘要
Treatment nonadherence in people with schizophrenia is associated with relapse and homelessness. Building on the usefulness of long-acting medication and our work in psychosocial interventions to enhance adherence, we conducted a prospective uncontrolled trial of customized adherence enhancement (CAE) plus long-acting injectable antipsychotic (LAI) using haloperidol decanoate in 30 homeless or recently homeless individuals with DSM-IV-defined schizophrenia or schizoaffective disorder.Participants received monthly CAE and LAI (CAE-L) for 6 months. Primary outcomes were adherence, as measured by the Tablets Routine Questionnaire, and housing status. Secondary outcomes included psychiatric symptoms, functioning, side effects, and hospitalizations. The study was conducted from July 2010 to December 2012.The mean age of participants was 41.8 years (SD = 8.6); they were mainly minorities (90%, n = 27 African-American) and mainly single/never married (70%, n = 21). Most (97%, n = 29) had past or current substance abuse and had been incarcerated (97%, n = 29). Ten individuals (33%) terminated the study prematurely. CAE-L was associated with good adherence to LAI (at 6 months, 76%) and dramatic improvement in oral medication adherence, which changed from missing 46% of medication at study enrollment to missing only 10% at study end (P = .03). There were significant improvements in psychiatric symptoms (P < .001) and functioning (P < .001). Akathisia was a major side effect with LAI.While interpretation of findings must be tempered by the methodological limitations, CAE-L appears to be associated with improved adherence, symptoms, and functioning in homeless or recently homeless individuals with schizophrenia or schizoaffective disorder. Additional research is needed on effective and practical approaches to improving health outcomes for homeless people with serious mental illness.ClinicalTrials.gov identifier: NCT01152697.© Copyright 2013 Physicians Postgraduate Press, Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
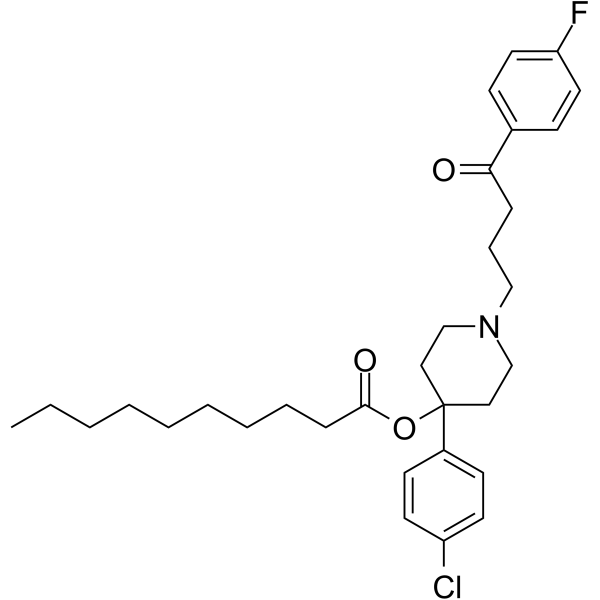 |
氟哌啶醇癸酸酯
CAS:74050-97-8 |
C31H41ClFNO3 |
|
Effectiveness of paliperidone palmitate vs haloperidol decan...
2014-05-21 [JAMA 311(19) , 1978-87, (2014)] |
|
Maintenance treatment with long-acting injectable antipsycho...
2014-05-21 [JAMA 311(19) , 1973-4, (2014)] |
|
Ten year outcomes of outpatients with schizophrenia on conve...
2013-09-01 [Int. Clin. Psychopharmacol. 28(5) , 261-6, (2013)] |
|
Effects of omega-3 essential fatty acids (omega-3 EFAs) on m...
2010-04-01 [Neurotox. Res. 17(3) , 228-37, (2010)] |
|
Differing effects of antipsychotic medications on substance ...
2006-01-01 [Am. J. Addict. 15(2) , 166-73, (2006)] |