Fluorescence spectrometric study on the interaction of tamibarotene with bovine serum albumin.
Huazhen Ye, Bin Qiu, Zhenyu Lin, Guonan Chen
文献索引:Luminescence 26(5) , 336-41, (2011)
全文:HTML全文
摘要
The interaction between tamibarotene and bovine serum albumin (BSA) was studied using fluorescence quenching technique and ultraviolet-visible spectrophotometry. The results of experiments showed that tamibarotene could strongly quench the intrinsic fluorescence of BSA by a dynamic quenching mechanism. The apparent binding constant, number of binding site and corresponding thermodynamic parameters at different temperatures were calculated respectively, and the main interaction force between tamibarotene and BSA was proved to be hydrophobic force. Synchronous fluorescence spectra showed that tamibarotene changed the molecular conformation of BSA. When BSA concentration was 1.00 × 10⁻⁶mol L⁻¹, the quenched fluorescence ΔF had a good linear relationship with the concentration of tamibarotene in the range 1.00 × 10⁻⁶ to 12.00 × 10⁻⁶ mol L⁻¹ with the detection limit of 6.52 × 10⁻⁷ mol L⁻¹.Copyright © 2010 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
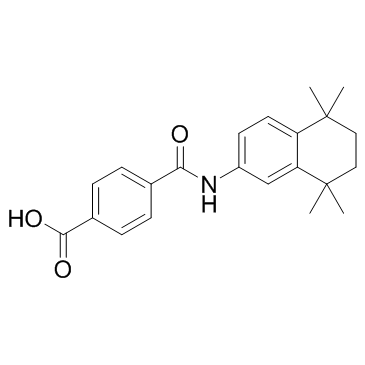 |
他米巴罗汀
CAS:94497-51-5 |
C22H25NO3 |
|
Retinoic acid acts as a selective human IgA switch factor.
2014-08-01 [Hum. Immunol. 75(8) , 923-9, (2014)] |
|
Retinoid signaling is necessary for, and promotes long-term ...
2014-10-01 [Neurobiol. Learn. Mem. 114 , 127-40, (2014)] |
|
Role of hematopoietic stem cell transplantation for relapsed...
2013-10-01 [Cancer Sci. 104(10) , 1339-45, (2013)] |
|
A high-content imaging-based screening pipeline for the syst...
2016-03-01 [Methods 96 , 46-58, (2016)] |
|
The Induction Effect of Am80 and TSA on ESC Differentiation ...
2015-01-01 [PLoS ONE 10 , e0140262, (2015)] |