Comparison of nicotinamide, ethylurea and polyethylene glycol as carriers for nifedipine solid dispersion systems.
H Suzuki, H Sunada
文献索引:Chem. Pharm. Bull. 45(10) , 1688-93, (1997)
全文:HTML全文
摘要
The most prevalent means for producing solid dispersions of nifedipine, a poorly water-soluble drug, are the solvent based processes that bring problems of environmental and health. We have investigated the preparation of solid dispersions of nifedipine (mp 173 degrees C) by the fusion method, using nicotinamide, ethylurea, polyethylene glycol (PEG) 6000 and hydroxypropylmethylcellulose (HPMC) as carriers. All these solid dispersions were obtained by cooling at room temperature after heating at 140 degrees C for 15 min. As a single carrier, nicotinamide, ethylurea and PEG were used because nifedipine dissolved in their fused liquids. Compared with the physical mixtures, the solid dispersions with ethylurea or PEG led to a higher dissolution rate of the drug, whereas the difference in drug release between the physical mixtures and the solid dispersions with nicotinamide was not clear. This peculiarity might be due to the high solubilizing effect of nicotinamide for the drug. The fused mixtures of nicotinamide-, ethylurea- or PEG-HPMC were utilized as combined carriers. HPMC dissolved in the fused liquids of nicotinamide or ethylurea, which was effective in forming the amorphous nifedipine in solid dispersions. This resulted in not only the enhanced dissolution rate but also the supersaturation behavior of nifedipine. Further, for the nicotinamide-HPMC system, the supersaturation level of nifedipine increased with an increase in the HPMC content of solid dispersions. Nicotinamide was more applicable than ethylurea and PEG for preparation of the fused dispersions of nifedipine.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
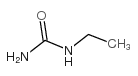 |
乙基脲
CAS:625-52-5 |
C3H8N2O |
|
Solvation of human serum albumin in aqueous alkylurea soluti...
1993-10-01 [Int. J. Pept. Protein Res. 42(4) , 320-5, (1993)] |
|
Inhibition of HIV-1 infection by alkylureas.
1991-12-01 [AIDS 5(12) , 1447-52, (1991)] |
|
Neoplasia induced in male rats fed lead acetate, ethyl urea,...
1985-01-01 [Toxicol. Pathol. 13(1) , 50-7, (1985)] |
|
Mutagenic effects of nitrogen dioxide combined with methylur...
1981-03-01 [Mutat. Res. 88(3) , 281-90, (1981)] |
|
Alterations in the reproductive performance of habrobracon f...
1981-02-01 [Mutat. Res. 88(2) , 179-89, (1981)] |