| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
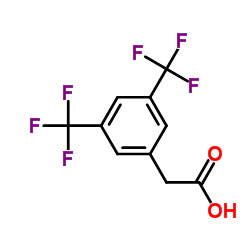 |
3,5-双(三氟甲基)苯乙酸
CAS:85068-33-3 |
|
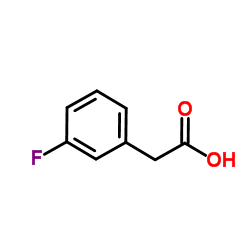 |
3-氟苯乙酸
CAS:331-25-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
3,5-双(三氟甲基)苯乙酸
CAS:85068-33-3 |
|
 |
3-氟苯乙酸
CAS:331-25-9 |