| Structure | Name/CAS No. | Articles |
|---|---|---|
![[3,5-Bis(trifluoromethyl)phenyl]acetic acid Structure](https://image.chemsrc.com/caspic/458/85068-33-3.png) |
[3,5-Bis(trifluoromethyl)phenyl]acetic acid
CAS:85068-33-3 |
|
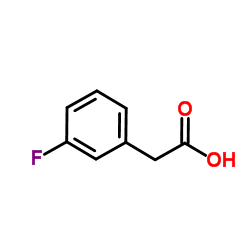 |
3-Fluorophenylacetic acid
CAS:331-25-9 |