Crystal structure of a conformation-selective casein kinase-1 inhibitor.
N Mashhoon, A J DeMaggio, V Tereshko, S C Bergmeier, M Egli, M F Hoekstra, J Kuret
文献索引:J. Biol. Chem. 275 , 20052-20060 , (2000)
全文:HTML全文
摘要
Members of the casein kinase-1 family of protein kinases play an essential role in cell regulation and disease pathogenesis. Unlike most protein kinases, they appear to function as constitutively active enzymes. As a result, selective pharmacological inhibitors can play an important role in dissection of casein kinase-1-dependent processes. To address this need, new small molecule inhibitors of casein kinase-1 acting through ATP-competitive and ATP-noncompetitive mechanisms were isolated on the basis of in vitro screening. Here we report the crystal structure of 3-[(2,4,6-trimethoxyphenyl) methylidenyl]-indolin-2-one (IC261), an ATP-competitive inhibitor with differential activity among casein kinase-1 isoforms, in complex with the catalytic domain of fission yeast casein kinase-1 refined to a crystallographic R-factor of 22.4% at 2.8 A resolution. The structure reveals that IC261 stabilizes casein kinase-1 in a conformation midway between nucleotide substrate liganded and nonliganded conformations. We propose that adoption of this conformation by casein kinase-1 family members stabilizes a delocalized network of side chain interactions and results in a decreased dissociation rate of inhibitor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
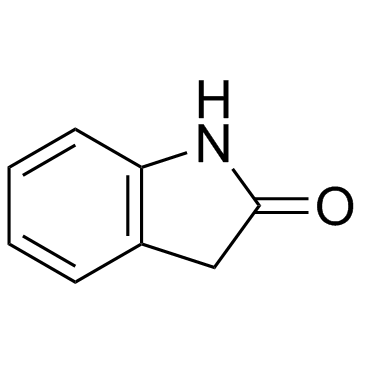 |
2-吲哚酮
CAS:59-48-3 |
C8H7NO |
|
Cytotoxic and antimicrobial evaluations of novel apoptotic a...
2015-02-01 [Arch. Pharm. (Weinheim) 348(2) , 113-24, (2015)] |
|
Synthesis and biological evaluation of new pyridone-annelate...
2014-01-01 [Molecules 19(9) , 13076-92, (2014)] |
|
Synthesis and receptor binding assay of indolin-2-one deriva...
2015-08-01 [Pharmazie 70 , 511-4, (2015)] |
|
Identification and Testing of Novel CARP-1 Functional Mimeti...
2015-09-01 [J. Biomed. Nanotechnol. 11 , 1608-27, (2015)] |
|
Construction of spiro-fused 2-oxindole/α-methylene- γ-butyro...
2013-12-20 [Org. Lett. 15(24) , 6182-5, (2013)] |