| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
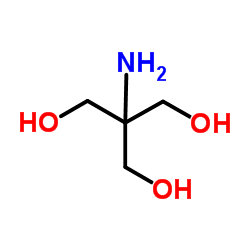 |
三(羟甲基)氨基甲烷
CAS:77-86-1 |
|
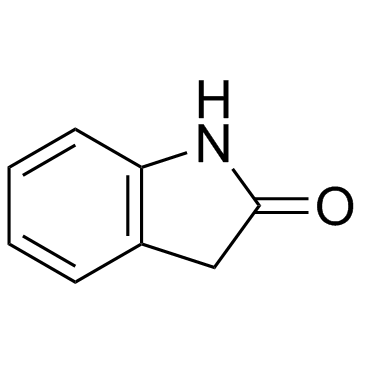 |
2-吲哚酮
CAS:59-48-3 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
三(羟甲基)氨基甲烷
CAS:77-86-1 |
|
 |
2-吲哚酮
CAS:59-48-3 |