| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
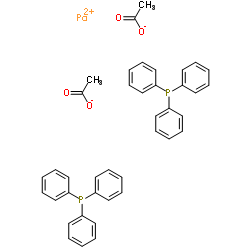 |
三苯基膦醋酸钯
CAS:14588-08-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
三苯基膦醋酸钯
CAS:14588-08-0 |