Superoxide dismutase-mediated reversible conversion of 3-hydroxyamino-1-methyl-5H-pyrido[4,3-b]indole, the N-hydroxy derivative of Trp-P-2, into its nitroso derivative.
K Hiramoto, K Negishi, T Namba, T Katsu, H Hayatsu
文献索引:Carcinogenesis 9(11) , 2003-8, (1988)
全文:HTML全文
摘要
Aerobic oxidation of 3-hydroxyamino-1-methyl-5H-pyrido-[4,3-b]indole [Trp-P-2(NHOH)] in neutral aqueous solution was greatly accelerated by copper-zinc superoxide dismutase (SOD). The major product in this SOD-mediated reaction was identified as 3-nitroso-1-methyl-5H-pyrido[4,3-b]indole [Trp-P-2(NO)]. This conversion was accompanied by a decrease of the mutagenicity of the mixture, as monitored by the direct-acting mutagenicity on Salmonella typhimurium TA98; a rapid change to approximately 1/3 of the original mutagenicity was followed by no further decrease of the activity. In contrast, in the spontaneous aerobic oxidation of Trp-P-2-(NHOH), the mutagenicity slowly and continuously decreased, until it was finally lost almost completely. Similar acceleration by SOD of aerobic oxidation was found for 2-hydroxyamino-6-methyldipyrido[1,2-a:3',2'-d]imidazole [Glu-P-1(NHOH)]. Again, mutagenicity of approximately 1/4 that of the original was retained in the SOD-mediated decomposition, while a complete loss of the mutagenicity was observed in the spontaneous decomposition. When Trp-P-2(NO) was treated with the superoxide-generating system, xanthine oxidase plus xanthine, Trp-P-2(NHOH) was formed. Therefore, the role of SOD in the conversion of Trp-P-2(NHOH) into Trp-P-2(NO) is the removal of superoxide anions generated by reduction of aerobic oxygen, thereby inhibiting the reverse reactions, i.e. the reduction of Trp-P-2(NO) and that of the putative intermediate nitroxide radical. In support of this proposed mechanism, phenylhydroxylamine underwent a SOD-accelerated conversion to nitrosobenzene, and nitrosobenzene was reduced to phenylhydroxylamine by the action of the xanthine oxidase-xanthine system. Hence, this reversible interchange between an arylhydroxylamine and its nitroso compound, coupled with the oxygen-superoxide cycle, may be a general phenomenon. A consequence of this finding is that the xenobiotic N-hydroxylamines may be converted by the action of SOD in the biological settings into nitroso compounds, which are chemically more stable, serving as a reservoir for mutagenicity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
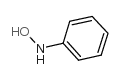 |
N-苯基羟胺
CAS:100-65-2 |
C6H7NO |
|
In situ infrared monitoring of the solid/liquid catalyst int...
2011-07-21 [Phys. Chem. Chem. Phys. 13(27) , 12463-71, (2011)] |
|
Genotoxic activities of aniline and its metabolites and thei...
2005-12-01 [Crit. Rev. Toxicol. 35(10) , 783-835, (2005)] |
|
Biotransformation of hydroxylaminobenzene and aminophenol by...
2000-06-01 [Appl. Environ. Microbiol. 66(6) , 2336-42, (2000)] |
|
[Phenoxenium ions: generations and reactions].
1994-08-01 [Yakugaku Zasshi 114(8) , 565-76, (1994)] |
|
Thrombelastographic characterization of coagulation/fibrinol...
2013-04-01 [Blood Coagul. Fibrinolysis 24(3) , 273-8, (2013)] |