Thiolysis of the 3-nitro-2-pyridinesulfenyl (Npys) protecting group. An approach towards a general deprotection scheme in peptide synthesis.
O Rosen, S Rubinraut, M Fridkin
文献索引:Int. J. Pept. Protein Res. 35(6) , 545-9, (1990)
全文:HTML全文
摘要
The hydroxylic side-chain functional groups of serine, threonine, hydroxproline and tyrosine, the alpha and epsilon-amino moieties of lysine and the thiol group of cysteine were masked by the 3-nitro-2-pyridinesulfenyl (Npys) protecting group. Deprotection was mildly affected by thiolysis with either 2-mercaptopyridine and 2-mercaptomethyl imidazole (O- and N-Npys) or with 3-mercaptoacetic acid and 2-mercaptoethanol (S-Npys). Thiolysis was monitored spectrophotometrically and was completed in a rather short time. Incorporation of the Npys group into a whole and single thiolyzable deprotection scheme is suggested.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
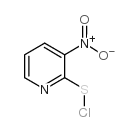 |
3-硝基-2-吡啶硫酰氯
CAS:68206-45-1 |
C5H3ClN2O2S |
|
Synthesis of disulfide-bridged fragments of omega-conotoxins...
1994-04-01 [Int. J. Pept. Protein Res. 43(4) , 363-6, (1994)] |
|
Synthesis and stability of 3-nitro-2-pyridinesulfenyl chlori...
1993-11-01 [Int. J. Pept. Protein Res. 42(2) , 159-64, (1993)] |
|
Discriminative affinity labelling of opioid receptors by enk...
[J. Chromatogr. A. 597(1-2) , 425-8, (1992)] |
|
Compatibility of the S-(3-nitro-2-pyridinesulfenyl) protecti...
1992-01-01 [Pept. Res. 5(5) , 262-4, (1992)] |
|
Design and synthesis of a kininogen-based selective inhibito...
1994-01-01 [Pept. Res. 7 , 32-35, (1994)] |