Discriminative affinity labelling of opioid receptors by enkephalin and morphiceptin analogues containing 3-nitro-2-pyridinesulphenyl-activated thiol residues.
Y Shimohigashi, K Takada, H Sakamoto, H Matsumoto, T Yasunaga, M Kondo, M Ohno
文献索引:J. Chromatogr. A. 597(1-2) , 425-8, (1992)
全文:HTML全文
摘要
The thiol groups of leucinthiol, cysteamine and cysteine incorporated into opioid peptides enkephalin and morphiceptin were activated by the 3-nitro-2-pyridinesulphenyl (Npys) group to form mixed disulphides highly reactive to a free thiol. Enkephalin analogues containing Npys-leucinthiol or -cysteine at positions 4, 5 and 6 exhibited high affinities for both mu and delta receptors, while morphiceptin analogues containing Npys-cysteine at positions 4 and 5 showed relatively weak affinity only for mu receptors. When these S-activated opioid peptides were incubated with rat brain membrane preparations, it was found, by binding assay using radiolabelled and non-labelled [D-Ala2,MePhe4,Gly-ol5]enkephalin, that they label mu opioid receptors in a dose-dependent manner. The concentrations required to label half of the receptors were 0.2-2 microM for enkephalins and 10-30 microM for morphiceptins. These results suggested that the thiol group labelled by S-activated enkephalins and morphiceptins is present in the ligand binding site of receptor protein, but not in GTPase-binding protein.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
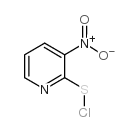 |
3-硝基-2-吡啶硫酰氯
CAS:68206-45-1 |
C5H3ClN2O2S |
|
Synthesis of disulfide-bridged fragments of omega-conotoxins...
1994-04-01 [Int. J. Pept. Protein Res. 43(4) , 363-6, (1994)] |
|
Synthesis and stability of 3-nitro-2-pyridinesulfenyl chlori...
1993-11-01 [Int. J. Pept. Protein Res. 42(2) , 159-64, (1993)] |
|
Compatibility of the S-(3-nitro-2-pyridinesulfenyl) protecti...
1992-01-01 [Pept. Res. 5(5) , 262-4, (1992)] |
|
Thiolysis of the 3-nitro-2-pyridinesulfenyl (Npys) protectin...
1990-06-01 [Int. J. Pept. Protein Res. 35(6) , 545-9, (1990)] |
|
Design and synthesis of a kininogen-based selective inhibito...
1994-01-01 [Pept. Res. 7 , 32-35, (1994)] |