Photosynthesis-Inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates.
Ales Imramovsky, Matus Pesko, Juana Monreal Ferriz, Katarina Kralova, Jarmila Vinsova, Josef Jampilek
文献索引:Bioorg. Med. Chem. Lett. 21(15) , 4564-7, (2011)
全文:HTML全文
摘要
A series of photosynthetic electron transport (PET) inhibitors from the group of salicylanilide alkylcarbamates was investigated. The compounds were analyzed using RP-HPLC to determine lipophilicity, and their PET inhibition was determined in spinach (Spinacia oleracea L.) chloroplasts. The site of action of the studied compounds is situated at the donor site of photosystem 2 (PS 2). Compounds substituted by chlorine in C'-3 and C'-4 of the aniline ring and the optimal length of the alkyl chain pentyl-heptyl in the carbamate moiety provided the most active PET inhibitors (IC(50) inhibition <10 μmol/L). Disubstitution in C'-3,4 by chlorine caused significant PET inhibiting activity decrease. Nevertheless, for all three series of C'-3, C'-4, C'-3,4 compounds, the dependence of PET activity on lipophilicity showed to be quasi-parabolic.Copyright © 2011 Elsevier Ltd. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
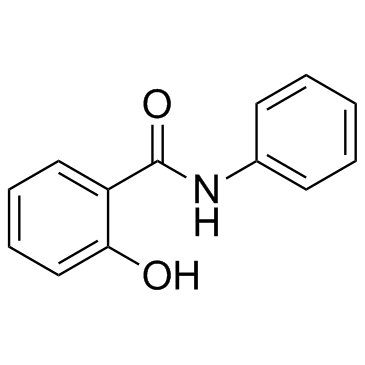 |
水杨酰苯胺
CAS:87-17-2 |
C13H11NO2 |
|
A closer study of peak distortions in supercritical fluid ch...
2015-06-26 [J. Chromatogr. A. 1400 , 131-9, (2015)] |
|
Application of reactive salicylanilide to viscose fabrics as...
2012-01-01 [Int. J. Biol. Macromol. 50(1) , 273-6, (2012)] |
|
Harmaline-resistant mutant of Methanothermobacter thermautot...
2011-01-01 [Gen. Physiol. Biophys. 30 Spec No , S54-60, (2011)] |
|
Salicylanilide carbamates: antitubercular agents active agai...
2010-02-01 [Bioorg. Med. Chem. 18 , 1054-61, (2010)] |
|
Salicylanilide esters of N-protected amino acids as novel an...
2009-01-15 [Bioorg. Med. Chem. Lett. 19 , 348-51, (2009)] |