Thermodynamic analysis of alcohol effect on thermal stability of proteins.
Osato Miyawaki, Michiko Tatsuno
文献索引:J. Biosci. Bioeng. 111(2) , 198-203, (2011)
全文:HTML全文
摘要
Thermal unfolding of ribonuclease A and α-chymotrypsinogen A was analyzed in various alcohol solutions of methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, tert-butanol, trifluoroethanol, and glycerol. The change in thermal unfolding ratio with temperature was described well by the van't Hoff equation and the melting temperature and the enthalpy of protein unfolding were obtained. The reciprocal form of the Wyman-Tanford equation, which describes the unfolded-to-folded protein ratio as a function of water activity, was applied to obtain a linear plot. From the slope of this plot and water activity, the stabilization free energy (ΔΔG) in a solution was calculated. This shows an important role of water activity in protein stability. ΔΔG was linearly dependent on alcohol concentration and m-values of alcohols for protein unfolding were obtained. This provides a theoretical basis for the linear extrapolation model (LEM). The m-values for alcohols were negative except for glycerol. The negative higher m-value for longer and linear chain alcohols suggested the important role of the disturbance of hydrophobic interactions as well as the hydrogen-bonding in the mechanism of protein destabilization by alcohols. The number of change in bound-alcohol molecules upon protein unfolding was also obtained.Copyright © 2010 The Society for Biotechnology, Japan. Published by Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
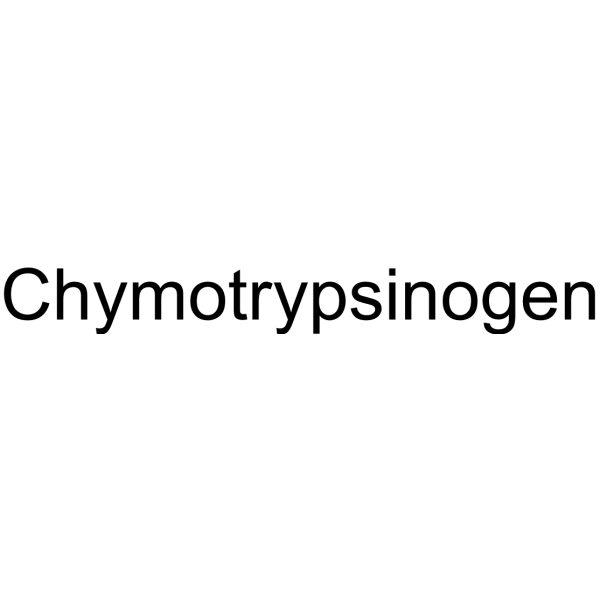 |
A-胰凝乳蛋白酶原
CAS:9035-75-0 |
|
Protein adsorption, desorption, and aggregation mediated by ...
2015-06-01 [J. Pharm. Sci. 104 , 1946-59, (2015)] |
|
Electrodynamic pressure modulation of protein stability in c...
2013-11-19 [Biochemistry 52(46) , 8363-73, (2013)] |
|
Pharmacological inhibition of PAR2 with the pepducin P2pal-1...
2014-03-01 [Am. J. Physiol. Gastrointest. Liver Physiol. 304(5) , G516-26, (2013)] |
|
Selecting temperature for protein crystallization screens us...
2011-01-01 [PLoS ONE 6 , e17950, (2011)] |
|
Using the water signal to detect invisible exchanging proton...
2011-08-01 [J. Biomol. NMR 50(4) , 299-314, (2011)] |