Biotechnology Letters
2010-01-01
Diastereoselective synthesis of L: -threo-3,4-dihydroxyphenylserine by low-specific L: -threonine aldolase mutants.
Hui-Jeong Gwon, Sang-Ho Baik
文献索引:Biotechnol. Lett. 32(1) , 143-9, (2010)
全文:HTML全文
摘要
Diastereoselectivity-enhanced mutants of L: -threonine aldolase (L: -TA) for L: -threo-3,4-dihydroxyphenylserine (L: -threo-DOPS) synthesis were isolated by error-prone PCR followed by a high-throughput screening. The most improved mutant was achieved from the mutant T3-3mm2, showing a 4-fold increase over the wild-type L: -TA. When aldol condensation activity was examined using whole cells of T3-3mm2, its de was constantly maintained at 55% during the batch reactions for 80 h, yielding 3.8 mg L: -threo-DOPS/ml.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
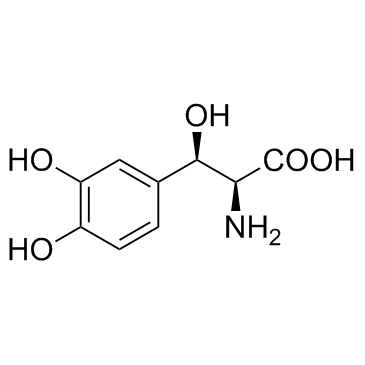 |
屈西多巴
CAS:23651-95-8 |
C9H11NO5 |
相关文献:
更多...
|
Application of the BCS biowaiver approach to assessing bioeq...
2014-11-20 [Eur. J. Pharm. Sci. 64 , 37-43, (2014)] |
|
[Acute poisoning of droxidopa: report of a case].
2013-09-01 [Chudoku. Kenkyu. 26(3) , 244-5, (2013)] |
|
The noradrenaline precursor L-DOPS reduces pathology in a mo...
2012-01-01 [Neurobiol. Aging 33(8) , 1651-63, (2012)] |
|
Comment on "Midodrine hydrochloride and L-threo-3,4-dihydrox...
2007-10-01 [Ther. Apher. Dial. 11(5) , 407-8, (2007)] |
|
Randomized withdrawal study of patients with symptomatic neu...
2015-01-01 [Hypertension 65(1) , 101-7, (2015)] |