| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
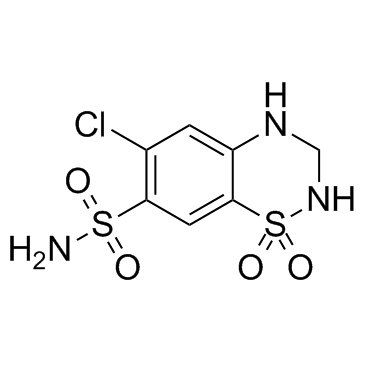 |
氢氯噻嗪
CAS:58-93-5 |
|
 |
法莫替丁
CAS:76824-35-6 |
|
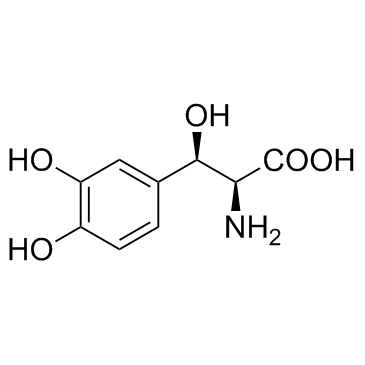 |
屈西多巴
CAS:23651-95-8 |