Metabolism of tetramethrin isomers in rat. III. Stereochemistry of reduced metabolites.
Y Tomigahara, M Onogi, M Miki, K Yanagi, K Shiba, H Kaneko, I Nakatsuka, H Yamada
文献索引:Xenobiotica 26(2) , 201-10, (1996)
全文:HTML全文
摘要
1. Three main urinary metabolites, two isomers of 3-hydroxycyclohexane-1,2-dicarboximide (3-OH-HPI-1 and 2) and 1,2-tetrahydrodicarboxylic acid (TCDA) were purified from rat treated with (1RS, trans)-tetramethrin [3,4,5,6-tetrahydrophthalimidomethyl (1RS, trans)-chrysanthemate]. 2. To elucidate the mechanism of formation of these reduced metabolites, the stereochemistry of 3-OH-HPI-1, 3-OH-HPI-2 and TCDA was clarified by chemical reactions, spectroanalysis (nmr) and X-ray analysis. 3. The sole difference in configuration between 3-OH-HPI-1 and 3-OH-HPI-2 was found to be the orientation of the hydroxyl group to the cyclohexane ring, and both of these reduced metabolites showed cis-addition of two hydrogens. In contrast, reduction resulted in the trans form with TCDA. 4. These findings indicate the existence of two different reduction reaction mechanisms in the rat.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
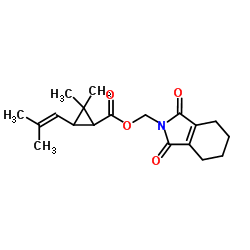 |
胺菊酯
CAS:7696-12-0 |
C19H25NO4 |
|
Characterisation of gene expression patterns in 22RV1 cells ...
2003-02-01 [J. Steroid Biochem. Mol. Biol. 84(2-3) , 231-8, (2003)] |
|
Indoor thermal fogging application of pesguard FG 161, a mix...
2001-03-01 [J. Am. Mosq. Control Assoc. 17(1) , 28-32, (2001)] |
|
Differential pharmacology of the cardiac anionic background ...
2007-08-27 [Eur. J. Pharmacol. 569(3) , 163-70, (2007)] |
|
Comparison of two partial least squares infrared spectrometr...
2007-01-16 [Anal. Chim. Acta 582(1) , 174-80, (2007)] |
|
Non-neurogenic contractions in isolated coronary arteries by...
1994-12-01 [Cardiovasc. Res. 28(12) , 1843-53, (1994)] |