Metabolism of tetramethrin isomers in rat. III. Stereochemistry of reduced metabolites.
Y Tomigahara, M Onogi, M Miki, K Yanagi, K Shiba, H Kaneko, I Nakatsuka, H Yamada
Index: Xenobiotica 26(2) , 201-10, (1996)
Full Text: HTML
Abstract
1. Three main urinary metabolites, two isomers of 3-hydroxycyclohexane-1,2-dicarboximide (3-OH-HPI-1 and 2) and 1,2-tetrahydrodicarboxylic acid (TCDA) were purified from rat treated with (1RS, trans)-tetramethrin [3,4,5,6-tetrahydrophthalimidomethyl (1RS, trans)-chrysanthemate]. 2. To elucidate the mechanism of formation of these reduced metabolites, the stereochemistry of 3-OH-HPI-1, 3-OH-HPI-2 and TCDA was clarified by chemical reactions, spectroanalysis (nmr) and X-ray analysis. 3. The sole difference in configuration between 3-OH-HPI-1 and 3-OH-HPI-2 was found to be the orientation of the hydroxyl group to the cyclohexane ring, and both of these reduced metabolites showed cis-addition of two hydrogens. In contrast, reduction resulted in the trans form with TCDA. 4. These findings indicate the existence of two different reduction reaction mechanisms in the rat.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
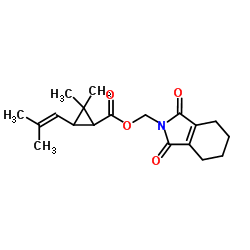 |
Tetramethrin
CAS:7696-12-0 |
C19H25NO4 |
|
Characterisation of gene expression patterns in 22RV1 cells ...
2003-02-01 [J. Steroid Biochem. Mol. Biol. 84(2-3) , 231-8, (2003)] |
|
Indoor thermal fogging application of pesguard FG 161, a mix...
2001-03-01 [J. Am. Mosq. Control Assoc. 17(1) , 28-32, (2001)] |
|
Differential pharmacology of the cardiac anionic background ...
2007-08-27 [Eur. J. Pharmacol. 569(3) , 163-70, (2007)] |
|
Comparison of two partial least squares infrared spectrometr...
2007-01-16 [Anal. Chim. Acta 582(1) , 174-80, (2007)] |
|
Non-neurogenic contractions in isolated coronary arteries by...
1994-12-01 [Cardiovasc. Res. 28(12) , 1843-53, (1994)] |