Site-specific modification or rabbit muscle aldolase with fluorescent probes.
P Dobryszycki, R Sakowicz, M Kochman
文献索引:Acta Biochim. Pol. 37(4) , 463-74, (1990)
全文:HTML全文
摘要
The site-specific modification of rabbit muscle aldolase A by labeling of thiol residues of Cys-289 with 5-(2-((iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid and Cys-239 with 5-iodoacetamidofluorescein or 4-dimethylamino-phenylazophenyl-4'-maleimide has been described. The method is based on the differences in kinetics of the chemical modification of aldolase thiols with the above reagents either in the presence or in the absence of a competitive inhibitor. The spectral properties of the doubly labeled aldolase derivatives were compared with those of the singly labeled enzyme. The doubly labeled aldolase derivatives exhibited full catalytic activity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
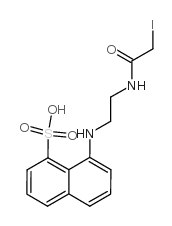 |
N-(碘乙酰氨基乙基)-8-萘胺-1-磺酸
CAS:36930-64-0 |
C14H15IN2O4S |
|
Conformational distributions and proximity relationships in ...
2000-01-28 [J. Biol. Chem. 275(4) , 2404-9, (2000)] |
|
Hairpin configuration of H-2Kk in liposomes formed by deterg...
1984-09-11 [Biochemistry 23(19) , 4401-9, (1984)] |
|
Assignment of free and disulfide-bonded cysteine residues in...
1996-07-23 [Biochemistry 35(29) , 9560-6, (1996)] |
|
Vectorially oriented monolayers of detergent-solubilized Ca(...
1996-05-01 [Biophys. J. 70(5) , 2131-43, (1996)] |
|
SH1 (cysteine 717) of smooth muscle myosin: its role in moto...
1999-09-07 [Biochemistry 38(36) , 11670-6, (1999)] |