Conformational distributions and proximity relationships in the rigor complex of actin and myosin subfragment-1.
M Nyitrai, G Hild, A Lukács, E Bódis, B Somogyi
文献索引:J. Biol. Chem. 275(4) , 2404-9, (2000)
全文:HTML全文
摘要
Cyclic conformational changes in the myosin head are considered essential for muscle contraction. We hereby show that the extension of the fluorescence resonance energy transfer method described originally by Taylor et al. (Taylor, D. L., Reidler, J., Spudich, J. A., and Stryer, L. (1981) J. Cell Biol. 89, 362-367) allows determination of the position of a labeled point outside the actin filament in supramolecular complexes and also characterization of the conformational heterogeneity of an actin-binding protein while considering donor-acceptor distance distributions. Using this method we analyzed proximity relationships between two labeled points of S1 and the actin filament in the acto-S1 rigor complex. The donor (N-[[(iodoacetyl)amino]ethyl]-5-naphthylamine-1-sulfonate) was attached to either the catalytic domain (Cys-707) or the essential light chain (Cys-177) of S1, whereas the acceptor (5-(iodoacetamido)fluorescein) was attached to the actin filament (Cys-374). In contrast to the narrow positional distribution (assumed as being Gaussian) of Cys-707 (5 +/- 3 A), the positional distribution of Cys-177 was found to be broad (102 +/- 4 A). Such a broad positional distribution of the label on the essential light chain of S1 may be important in accommodating the helically arranged acto-myosin binding relative to the filament axis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
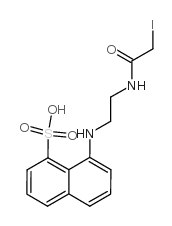 |
N-(碘乙酰氨基乙基)-8-萘胺-1-磺酸
CAS:36930-64-0 |
C14H15IN2O4S |
|
Hairpin configuration of H-2Kk in liposomes formed by deterg...
1984-09-11 [Biochemistry 23(19) , 4401-9, (1984)] |
|
Assignment of free and disulfide-bonded cysteine residues in...
1996-07-23 [Biochemistry 35(29) , 9560-6, (1996)] |
|
Vectorially oriented monolayers of detergent-solubilized Ca(...
1996-05-01 [Biophys. J. 70(5) , 2131-43, (1996)] |
|
SH1 (cysteine 717) of smooth muscle myosin: its role in moto...
1999-09-07 [Biochemistry 38(36) , 11670-6, (1999)] |
|
Replacement of ATP with ADP affects the dynamic and conforma...
1999-09-28 [Biochemistry 38(39) , 12885-92, (1999)] |