Stereoselective formation of carbon-carbon bonds via SN2-displacement: synthesis of substituted cycloalkyl[b]indoles.
Michael C Hillier, Jean-François Marcoux, Dalian Zhao, Edward J J Grabowski, Arlene E McKeown, Richard D Tillyer
文献索引:J. Org. Chem. 70(21) , 8385-94, (2005)
全文:HTML全文
摘要
A general asymmetric synthesis of substituted cycloalkyl[b]indoles has been accomplished. The key features of this approach are (1) the utilization of a Japp-Klingemann condensation/Fischer cyclization to prepare cycloalkyl[b]indolones, (2) the asymmetric borane reduction of these heterocyclic ketones with (S)-OAB to obtain enantiomerically pure alcohols, and (3) the stereoselective S(N)2-displacement of these indole alcohol substrates with a carbon nucleophile under Mitsunobu conditions to set the C1 or C3 tertiary carbon stereocenter. The use of trimethylphosphine (PMe3) and bis(2,2,2-trichloroethyl) azodicarboxylate (TCEAD) was found to have an effect on the Mitsunobu dehydrative alkylation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
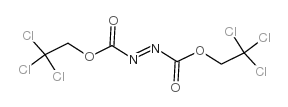 |
偶氮基二羧酸双(2,2,2-三氯乙酯)
CAS:38857-88-4 |
C6H4Cl6N2O4 |
|
Catalytic and enantioselective aza-ene and hetero-Diels-Alde...
2005-06-21 [Org. Biomol. Chem. 3(12) , 2344-9, (2005)] |
|
A convenient synthesis of N-acetyllactosamine derivatives fr...
1993-09-02 [Carbohydr. Res. 247 , 159-64, (1993)] |
|
N. Boudreault, Y. Leblanc
[Organic Synth. 74 , 241, (1997)] |
|
R.D. Little et al.
[J. Am. Chem. Soc. 101 , 7129, (1979)] |
|
M. Kaname et al.
[Tetrahedron Lett. 40 , 7993 , (1999)] |