| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
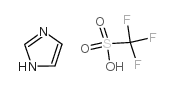 |
咪唑 三氟甲磺酸盐
CAS:29727-06-8 |
|
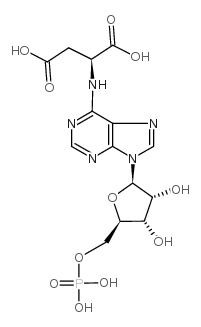 |
腺苷酸基琥珀酸
CAS:19046-78-7 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
咪唑 三氟甲磺酸盐
CAS:29727-06-8 |
|
 |
腺苷酸基琥珀酸
CAS:19046-78-7 |