Structural features of proinsulin C-peptide oligomeric and amyloid states.
Jesper Lind, Emma Lindahl, Alex Perálvarez-Marín, Anna Holmlund, Hans Jörnvall, Lena Mäler
文献索引:FEBS J. 277 , 3759-3768, (2010)
全文:HTML全文
摘要
The formation and structure of proinsulin C-peptide oligomers has been investigated by PAGE, NMR spectroscopy and dynamic light scattering. The results obtained show that C-peptide forms oligomers of different sizes, and that their formation and size distribution is altered by salt and divalent metal ions, which indicates that the aggregation process is mediated by electrostatic interactions. It is further demonstrated that the size distribution of the C-peptide oligomers, in agreement with previous studies, is altered by insulin, which supports a physiologically relevant interaction between these two peptides. A small fraction of oligomers has previously been suggested to be in equilibrium with a dominant fraction of soluble monomers, and this pattern also is observed in the present study. The addition of modest amounts of sodium dodecyl sulphate at low pH increases the relative amount of oligomers, and this effect was used to investigate the details of both oligomer formation and structure by a combination of biophysical techniques. The structural properties of the SDS-induced oligomers, as obtained by thioflavin T fluorescence, CD spectroscopy and IR spectroscopy, demonstrate that soluble aggregates are predominantly in β-sheet conformation, and that the oligomerization process shows characteristic features of amyloid formation. The formation of large, insoluble, β-sheet amyloid-like structures will alter the equilibrium between monomeric C-peptide and oligomers. This leads to the conclusion that the oligomerization of C-peptide may be relevant also at low concentrations.© 2010 The Authors Journal compilation © 2010 FEBS.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
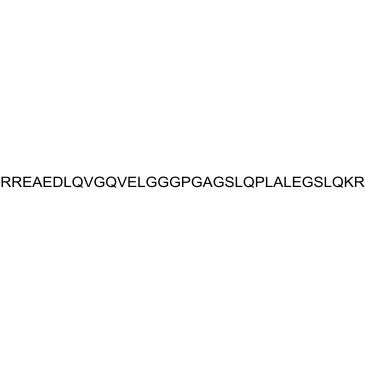 |
Proinsulin C-Peptide (55-89), human
CAS:11097-48-6 |
C153H259N49O52 |
|
Molecular and cellular effects of C-peptide--new perspective...
2004-01-01 [Exp. Diabesity Res. 5 , 15-23, (2004)] |
|
Cellular mechanisms by which proinsulin C-peptide prevents i...
2010-08-01 [Diabetologia 53 , 1761-71 , (2010)] |
|
Studies on human proinsulin. Isolation and amino acid sequen...
1971-03-10 [J. Biol. Chem. 246 , 1375, (1971)] |
|
N-terminal segment of proinsulin C-peptide active in insulin...
2010-01-01 [Biochem. Biophys. Res. Commun. 403 , 462-467, (2010)] |
|
Intracellular signaling by C-peptide. Hills, C.E. and Brunsk...
[Exp. Diabetes Res. , 635158, (2008)] |