Adenosine analogues as selective inhibitors of glyceraldehyde-3-phosphate dehydrogenase of Trypanosomatidae via structure-based drug design.
J C Bressi, C L Verlinde, A M Aronov, M L Shaw, S S Shin, L N Nguyen, S Suresh, F S Buckner, W C Van Voorhis, I D Kuntz, W G Hol, M H Gelb
文献索引:J. Med. Chem. 44(13) , 2080-93, (2001)
全文:HTML全文
摘要
In our continuation of the structure-based design of anti-trypanosomatid drugs, parasite-selective adenosine analogues were identified as low micromolar inhibitors of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Crystal structures of Trypanosoma brucei, Trypanosoma cruzi, Leishmania mexicana, and human GAPDH's provided details of how the adenosyl moiety of NAD(+) interacts with the proteins, and this facilitated the understanding of the relative affinities of a series of adenosine analogues for the various GAPDH's. From exploration of modifications of the naphthalenemethyl and benzamide substituents of a lead compound, N(6)-(1-naphthalenemethyl)-2'-deoxy-2'-(3-methoxybenzamido)adenosine (6e), N(6)-(substituted-naphthalenemethyl)-2'-deoxy-2'-(substituted-benzamido)adenosine analogues were investigated. N(6)-(1-Naphthalenemethyl)-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (6m), N(6)-[1-(3-hydroxynaphthalene)methyl]-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (7m), N(6)-[1-(3-methoxynaphthalene)methyl]-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (9m), N(6)-(2-naphthalenemethyl)-2'-deoxy-2'-(3-methoxybenzamido)adenosine (11e), and N(6)-(2-naphthalenemethyl)-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (11m) demonstrated a 2- to 3-fold improvement over 6e and a 7100- to 25000-fold improvement over the adenosine template. IC(50)'s of these compounds were in the range 2-12 microM for T. brucei, T. cruzi, and L. mexicana GAPDH's, and these compounds did not inhibit mammalian GAPDH when tested at their solubility limit. To explore more thoroughly the structure-activity relationships of this class of compounds, a library of 240 N(6)-(substituted)-2'-deoxy-2'-(amido)adenosine analogues was generated using parallel solution-phase synthesis with N(6) and C2' substituents chosen on the basis of computational docking scores. This resulted in the identification of 40 additional compounds that inhibit parasite GAPDH's in the low micromolar range. We also explored adenosine analogues containing 5'-amido substituents and found that 2',5'-dideoxy-2'-(3,5-dimethoxybenzamido)-5'-(diphenylacetamido)adenosine (49) displays an IC(50) of 60-100 microM against the three parasite GAPDH's.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
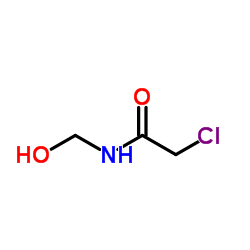 |
N-羟甲基氯乙酰胺
CAS:2832-19-1 |
C3H6ClNO2 |
|
Formaldehyde-releasers: relationship to formaldehyde contact...
2010-09-01 [Contact Dermatitis 63(3) , 129-39, (2010)] |
|
Formaldehyde-releasers: relationship to formaldehyde contact...
2009-08-01 [Contact Dermatitis 61(2) , 63-85, (2009)] |
|
Effect of Prolonged Repeated Exposure to Formaldehyde Donors...
2007-07-01 [J. Immunotoxicol. 4(3) , 239-46, (2007)] |
|
Occupational contact dermatitis to N-methylol-chloracetamide...
1987-09-01 [Contact Dermatitis 17(3) , 182-4, (1987)] |
|
Allergic contact dermatitis from the preservative N-methylol...
1980-06-01 [Contact Dermatitis 6(4) , 302-3, (1980)] |