| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
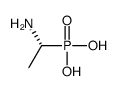 |
(R)-(-)-1-氨乙基膦酸
CAS:60687-36-7 |
|
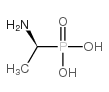 |
(S)-(+)-1-氨基乙基膦酸
CAS:66068-76-6 |