| Structure | Name/CAS No. | Articles |
|---|---|---|
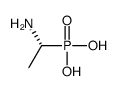 |
(R)-(1-Aminoethyl)phosphonic acid
CAS:60687-36-7 |
|
 |
(S)-(-)-PROPRANOLOLHYDROCHLORIDE
CAS:66068-76-6 |