| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
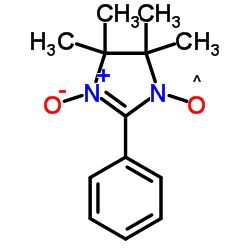 |
3-氧代-2-苯基-4,4,5,5-四甲基咪唑啉-1-氧
CAS:18390-00-6 |
|
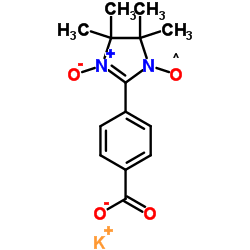 |
羧基-PTIO 钾盐
CAS:148819-94-7 |