Photoaffinity reagents for use with pepsin and other carboxyl proteases.
S H Hixson, J L Hurwitz, K J Langridge, D C Nichols, K M Provost, A M Wolff
文献索引:Biochem. Biophys. Res. Commun. 111(2) , 630-5, (1983)
全文:HTML全文
摘要
Two compounds have been designed to serve as photoaffinity reagents for use with carboxyl proteases. 1,2-Epoxy-3-(4'-azido-2'-nitrophenoxy)propane has been synthesized and shown to react with porcine pepsin in the same fashion as the traditional inhibitor 1,2-epoxy-3-(p-nitrophenoxy)propane, while p-azidophenacyl bromide is similar to other phenacyl bromides in its reaction with pepsin. In combination with p-azido-alpha-diazoacetophenone, previously shown to resemble alpha-diazo carbonyl reagents in its reaction with pepsin, photoaffinity analogs are now available for all three of the widely-used carboxyl protease inhibitors.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
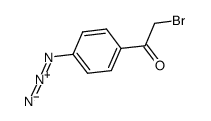 |
4-叠氮苯甲酰甲基溴
CAS:57018-46-9 |
C8H6BrN3O |
|
Direct identification of on-bead peptides using surface-enha...
2015-01-01 [Sci. Rep. 5 , 10144, (2015)] |
|
The σ70 region 1.2 regulates promoter escape by unwinding DN...
2013-04-01 [Nucleic Acids Res. 41(8) , 4565-72, (2013)] |
|
4-Bromophenacyl bromide specifically inhibits rhoptry secret...
2009-01-01 [PLoS ONE 4(12) , e8143, (2009)] |
|
Association of thioredoxin with the inner membrane and adhes...
1987-06-01 [J. Bacteriol. 169(6) , 2659-66, (1987)] |
|
Crosslinking of phenylalanyl-tRNA to the ribosomal A site vi...
1984-01-05 [J. Mol. Biol. 172(1) , 77-86, (1984)] |