| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
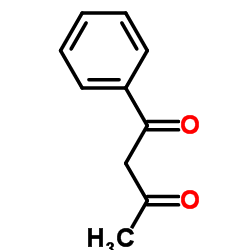 |
1-苯基-1,3-丁二酮
CAS:93-91-4 |
|
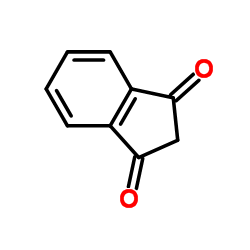 |
1,3-茚满二酮
CAS:606-23-5 |
|
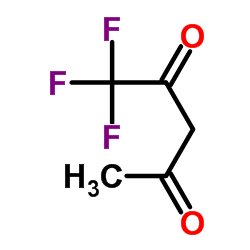 |
1,1,1-三氟乙酰丙酮
CAS:367-57-7 |