Anisotropic and hydrogen bonding effects in phenylglyoxamides and mandelamides: theoretical and NMR conformational evaluation.
Biank T Gonçalves, Pierre M Esteves, Angelo C Pinto, Carlos R Kaiser, Fernanda L da Silva, Eduardo Miguez, Joaquim F M da Silva
文献索引:Magn. Reson. Chem. 46(5) , 418-26, (2008)
全文:HTML全文
摘要
Interesting anisotropic effects were observed for phenylglyoxamides and their respective mandelamides. Such effects were observed in experimental (1)H and (13)C NMR (in CDCl(3), CD(3)OD, and DMSO-d(6) solvents) and in some cases with good correlation to theoretical (1)H and (13)C NMR DFT-GIAO (B3LYP/6-311++G**//B3LYP/6-31G*) calculations. A systematic conformational analysis of these compounds was performed in a two-step methodology, using PM3 and DFT (B3LYP/6-31G*) calculations; with good accomplishment and computational time economy. It was observed that intramolecular hydrogen bonding plays a significant role in the conformation of such compounds. Finally, a geminal nonequivalence of an N-CH(2) moiety, in one of the alkyl side chain (R1 = R2), was found for the tertiary mandelamides studied.2008 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
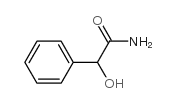 |
2-羟基-2-苯基乙酰胺
CAS:4410-31-5 |
C8H9NO2 |
|
The mandelamide keto-enol system in aqueous solution. Genera...
2003-01-08 [J. Am. Chem. Soc. 125(1) , 187-94, (2003)] |
|
[Molecular biology aspects of the antimicrobial effect of sy...
1982-08-01 [Z. Arztl. Fortbild. (Jena.) 76(15) , 662-6, (1982)] |
|
Catalytic asymmetric addition of dimethylzinc to alpha-ketoe...
2006-03-30 [Org. Lett. 8(7) , 1287-90, (2006)] |
|
Synthesis and fungicidal activity of N-2-(3-methoxy-4-propar...
2006-05-01 [Pest Manag. Sci. 62(5) , 446-51, (2006)] |
|
Synthesis and fungicidal activity of N-2-(3-methoxy-4-propar...
2007-01-01 [Pest Manag. Sci. 63(1) , 57-62, (2007)] |