| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
半胆碱-3
CAS:312-45-8 |
|
 |
5-氨基-3-(4-吗啉基)-1,2,3-恶二唑盐酸盐
CAS:16142-27-1 |
|
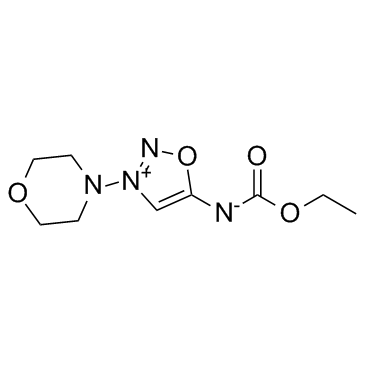 |
吗多明
CAS:25717-80-0 |