Determination of elastase inhibitory activity of alpha 1-proteinase inhibitor and bronchial antileukoprotease: different results using insoluble elastin or synthetic low molecular weight substrates.
J A Kramps, H M Morrison, D Burnett, J H Dijkman, R A Stockley
文献索引:Scand. J. Clin. Lab. Invest. 47(4) , 405-10, (1987)
全文:HTML全文
摘要
We have determined the effect of two elastase-specific synthetic low molecular weight substrates, L-pyroglutamylprolylvaline-paranitroanilide and succinyltrialanyl-paranitroanilide, together with insoluble elastin-fluorescein, on the determination of the neutrophil elastase (NE) inhibitory capacity of purified alpha 1-proteinase inhibitor (alpha 1-PI) and bronchial antileukoprotease (ALP). In addition, the inhibitory capacities of mixtures of alpha 1-proteinase inhibitor, antileukoprotease and alpha 2-macroglobulin prepared in ratios similar to that in lung secretions were determined. Purified inhibitors, alone or in combinations, inhibited about 1 mole neutrophil elastase per mole inhibitor when assessed using synthetic substrates. However, when elastin-fluorescein was used in the assay system, the purified inhibitors showed an inhibitory capacity that was 40-85% of the value obtained using synthetic substrates. Even less inhibition was observed when mixtures of inhibitors were assessed using elastin-fluorescein (23-44% of the value for synthetic substrates). Our data indicate that results of elastase inhibitor activity measurements depend on the type of substrate which has been used.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
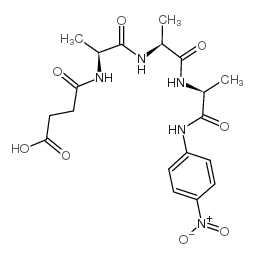 |
N-琥珀酰-L-丙氨酰-L-丙氨酰-L-丙氨酸
CAS:52299-14-6 |
C19H25N5O8 |
|
A new Kunitz-type plasmin inhibitor from scorpion venom.
2015-11-01 [Toxicon 106 , 7-13, (2015)] |
|
SjAPI, the first functionally characterized Ascaris-type pro...
2013-01-01 [PLoS ONE 8(3) , e57529, (2013)] |
|
Purification, kinetical and molecular characterizations of a...
1996-09-01 [Comp. Biochem. Physiol. B Biochem. Mol. Biol. 115(1) , 87-95, (1996)] |
|
Characterization of metalloelastase-like activity from the p...
1991-12-01 [Biol. Chem. Hoppe-Seyler 372 , 1057-1064, (1991)] |
|
Determination and characterization of succinyl tri-alanine p...
1987-01-01 [Enzyme 37(4) , 202-7, (1987)] |