Biochemical evidence for oligomerization of rat adrenal acyl-coenzyme A:cholesterol acyltransferase.
T Kawasaki, A Miyazaki, H Hakamata, H Matsuda, S Horiuchi
文献索引:Biochem. Biophys. Res. Commun. 244(2) , 347-52, (1998)
全文:HTML全文
摘要
Acyl-coenzyme A:cholesterol acyltransferase (ACAT) in rat adrenal was compared with that in rat liver. Immunoblot analyses of the microsomal fractions from adrenal with an anti-human ACAT antibody detected a 45 kDa protein. Upon pretreatment of these microsomal fractions with chemical cross-linkers such as BS3 and Sulfo-EGS, the 45 kDa band decreased with a concomitant increase in high molecular weight proteins (55, approximately 100, and approximately 230 kDa), suggesting that ACAT constitutes oligomers of 45 kDa monomers associated with a 10 kDa protein. In sharp contrast, the same immunoblot analysis of rat liver microsomal fractions identified a 50 kDa protein which was not cross-linked by these cross-linkers. Moreover, when four ACAT inhibitors were tested for their effects on adrenal and liver enzymes, NTE-122, CI-976, and E5324 were more effective for the liver enzyme, whereas 58-035 was much more effective for adrenal ACAT. These biochemical and pharmacological observations support the notion that the rat liver ACAT protein is distinct from the adrenal counterpart.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
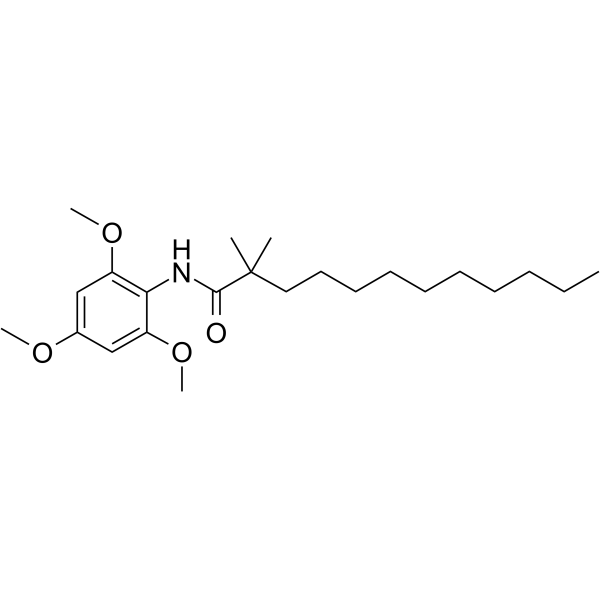 |
2,2-二甲基-N-(2,4,6-三甲氧基苯基)月桂酰胺
CAS:114289-47-3 |
C23H39NO4 |
|
cPLA2α and EHD1 interact and regulate the vesiculation of ch...
2012-05-01 [Mol. Biol. Cell 23(10) , 1874-88, (2012)] |
|
Use of acyltransferase inhibitors to block vesicular traffic...
2005-01-01 [Meth. Enzymol. 404 , 115-25, (2005)] |
|
A unique lysophospholipid acyltransferase (LPAT) antagonist,...
2005-07-15 [J. Cell Sci. 118(Pt 14) , 3061-71, (2005)] |
|
In vitro and in vivo disposition of 2,2-dimethyl-N-(2,4,6-tr...
1997-01-01 [Drug Metab. Dispos. 25(1) , 123-30, (1997)] |
|
The lysophospholipid acyltransferase antagonist CI-976 inhib...
2008-05-01 [Traffic 9(5) , 786-97, (2008)] |