Bile salt roles in bile-salt-stimulated lipase activity.
C J O'Connor, P Walde, R G Wallace
文献索引:J. Pediatr. Gastroenterol. Nutr. 5(4) , 622-9, (1986)
全文:HTML全文
摘要
The hydrolysis of 4-nitrophenylacetate and phenylsalicylate, catalyzed by human milk lipase in the presence of a range of concentrations of sodium cholate, has been measured at pH 7.3 and 37.5 degrees C, and maximum activity was observed for both substrates at 1 mmole/dm-3 bile salt. Lineweaver-Burk plots for the enzyme-catalyzed hydrolysis of N-methylindoxyl myristate and 4-nitrophenyloctanoate yielded values of Km equal to 34 and 20 mumoles/dm-3, respectively. However, an increase in the concentration of the latter substrate beyond 10 mumoles/dm-3 was not accompanied by a corresponding increase in the rate of hydrolysis. Comparison of these hydrolysis data with literature data for a variety of hydrophobic substrates suggests that there are two roles for the bile salt in the enzyme-catalyzed reaction--the first involving binding onto several sites on the enzyme, and the second solubilization of oil-phase substrates.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
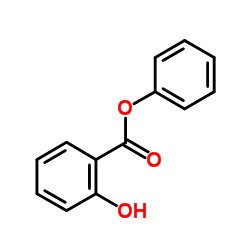 |
水杨酸苯酯
CAS:118-55-8 |
C13H10O3 |
|
Leaching of additives and degradation products from cold-cur...
1994-06-01 [Acta Odontol. Scand. 52(3) , 150-6, (1994)] |
|
In vitro transformation of hamster embryo cells by 3-(N-sali...
1981-01-01 [Toxicol. Lett. 7(3) , 211-5, (1981)] |
|
Fragrance material review on phenyl salicylate.
2007-01-01 [Food Chem. Toxicol. 45 Suppl 1 , S472-6, (2007)] |
|
Degradation kinetics of hydrolytically susceptible drugs in ...
2007-09-05 [Int. J. Pharm. 342(1-2) , 62-71, (2007)] |
|
Infrared spectroscopy study of structural changes in glass-f...
2010-03-01 [Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 81(3 Pt 1) , 031503, (2010)] |