The crystal structure of plant-specific calcium-binding protein AtCBL2 in complex with the regulatory domain of AtCIPK14.
Mayuko Akaboshi, Hiroshi Hashimoto, Hanako Ishida, Shinya Saijo, Nozomu Koizumi, Mamoru Sato, Toshiyuki Shimizu
文献索引:J. Mol. Biol. 377(1) , 246-57, (2008)
全文:HTML全文
摘要
Calcium signals mediate a multitude of plant responses to external stimuli. Calcineurin B-like (CBL) proteins and their target kinases, CBL-interacting protein kinases (CIPKs), represent important relays in plant calcium signaling. CBL interacts with CIPK through a conserved motif (NAF/FISL motif) within the C-terminal regulatory domain. To better understand the functional role of the CBL-CIPK system, we determined the crystal structure of AtCBL2 in complex with the regulatory domain of AtCIPK14 at 1.2 A resolution. The NAF/FISL motif is inserted into a hydrophobic crevice within AtCBL2, accompanied by a large displacement of the helices and loop on the opposite side of the NAF/FISL motif from the C-terminal region, which shields the hydrophobic crevice in free form. Ca(2+) are coordinated within four EF hands in AtCBL2 in bound form. This calcium coordination pattern differs from that in the structure of the SOS3-SOS2 complex previously reported. Structural comparison of the two structures shows that the recognition of CBL by CIPK is performed in a similar manner, but inherent interactions confer binding affinity and specificity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
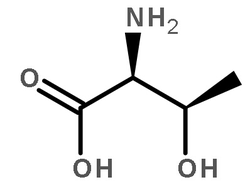 |
蛋白激酶A 来源于牛心脏
CAS:9026-43-1 |
C4H9NO3 |
|
Expression and localization of five members of the testis-sp...
2011-01-01 [Mol. Hum. Reprod. 17(1) , 42-56, (2011)] |
|
Hipk is an essential protein that promotes Notch signal tran...
2009-01-01 [Dev. Biol. 325(1) , 263-72, (2009)] |
|
Drosophila Smt3 negatively regulates JNK signaling through s...
2011-06-01 [Development 138(12) , 2477-85, (2011)] |
|
CIPK9: a calcium sensor-interacting protein kinase required ...
2007-05-01 [Cell Res. 17(5) , 411-21, (2007)] |
|
Tubulin binds to the cytoplasmic loop of TRESK background K⁺...
2014-01-01 [PLoS ONE 9(5) , e97854, (2014)] |