Liposomal muramyl tripeptide (CGP 19835A lipid) therapy for resectable melanoma in patients who were at high risk for relapse: an update.
M A Gianan, E S Kleinerman
文献索引:Cancer Biother. Radiopharm. 13(5) , 363-8, (1998)
全文:HTML全文
摘要
Liposome-encapsulated muramyl tripeptide-phosphatidyl ethanolamine (L-MTP-PE) was used in a pilot study for resectable melanoma patients who were at high risk for relapse. We entered 18 evaluable patients. The patient group included: (a) patients with stage III disease and clinically measurable regional metastases at presentation as confirmed by needle biopsy and (b) patients with stage IV disease presenting with measurable and resectable distant metastases confirmed by needle biopsy and limited to lungs, lymph nodes and subcutaneous tissues. L-MTP-PE was given for 4 weeks prior to surgical resection and for an additional 20 weeks postoperatively. Disease-free intervals were then determined based on the date of surgery. A preliminary report published in 1993 indicated an average disease-free interval of 18 months (range 8-33 months). This article presents an updated report on the long-term, disease-free survival status of these patients and shows that of the 18 evaluable patients, 4 remain free of disease for more than 5 years after surgical resection and therapy. The period of survival for these patients ranged from 69 months to more than 91 months (average 80.5 months). Although this was only a pilot study, we believe that the duration of survival indicates that L-MTP-PE may produce significant biologic activity in patients with melanoma, resulting in long-term benefits in terms of tumor eradication.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
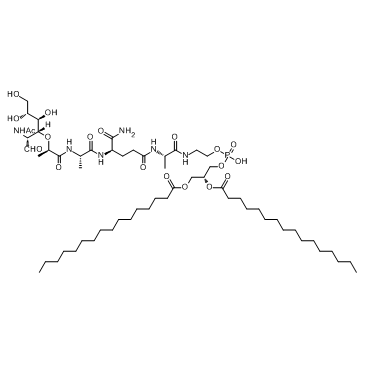 |
米法莫肽
CAS:83461-56-7 |
C59H109N6O19P |
|
Muramyl tripeptide (mifamurtide) for the treatment of osteos...
2009-08-01 [Expert Rev. Anticancer Ther. 9(8) , 1035-49, (2009)] |
|
Imunomodulative effect of liposomized muramyltripeptide phos...
2008-09-01 [Parasitol. Res. 103(4) , 919-29, (2008)] |
|
In vitro and in vivo enhancement of canine pulmonary alveola...
1999-04-01 [Cancer Biother. Radiopharm. 14(2) , 121-8, (1999)] |
|
Effect of MTP on TNF-alpha in perfused rat liver after bacte...
1998-05-01 [J. Surg. Res. 76(2) , 179-84, (1998)] |
|
Liposomal muramyl tripeptide phosphatidyl ethanolamine: a sa...
2008-02-01 [Expert Rev. Anticancer Ther. 8(2) , 151-9, (2008)] |