Humanization of excretory pathway in chimeric mice with humanized liver.
Hirotoshi Okumura, Miki Katoh, Toshiro Sawada, Miki Nakajima, Yoshinori Soeno, Hikaru Yabuuchi, Toshihiko Ikeda, Chise Tateno, Katsutoshi Yoshizato, Tsuyoshi Yokoi
文献索引:Toxicol. Sci. 97(2) , 533-8, (2007)
全文:HTML全文
摘要
The liver of a chimeric urokinase-type plasminogen activator (uPA)(+/+)/severe combined immunodeficient (SCID) mouse line recently established in Japan could be replaced by more than 80% with human hepatocytes. We previously reported that the chimeric mice with humanized liver could be useful as a human model in studies on drug metabolism and pharmacokinetics. In the present study, the humanization of an excretory pathway was investigated in the chimeric mice. Cefmetazole (CMZ) was used as a probe drug. The CMZ excretions in urine and feces were 81.0 and 5.9% of the dose, respectively, in chimeric mice and were 23.7 and 59.4% of the dose, respectively, in control uPA(-/-)/SCID mice. Because CMZ is mainly excreted in urine in humans, the excretory profile of chimeric mice was demonstrated to be similar to that of humans. In the chimeric mice, the hepatic mRNA expression of human drug transporters could be quantified. On the other hand, the hepatic mRNA expression of mouse drug transporters in the chimeric mice was significantly lower than in the control uPA(-/-)/SCID mice. In conclusion, chimeric mice exhibited a humanized profile of drug excretion, suggesting that this chimeric mouse line would be a useful animal model in excretory studies.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
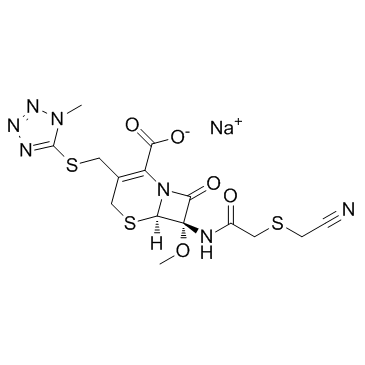 |
头孢美唑钠
CAS:56796-39-5 |
C15H16N7NaO5S3 |
|
Chimeric mice with humanized liver.
2008-04-03 [Toxicology 246(1) , 9-17, (2008)] |
|
Usefulness of sennoside as an agent for mechanical bowel pre...
2012-04-01 [Asian J. Surg. 35(2) , 81-7, (2012)] |
|
Involvement of multidrug resistance-associated protein 4 in ...
2010-06-01 [J. Pharmacol. Exp. Ther. 333 , 912-919, (2010)] |
|
Verification of cefmetazole and cefpodoxime proxetil contami...
2006-10-01 [Chem. Pharm. Bull. 54(10) , 1469-72, (2006)] |
|
Effectiveness of multimodal pain management after bipolar he...
2013-02-20 [J. Bone Joint Surg. Am. 95(4) , 291-6, (2013)] |